针对中高危前列腺癌的局部前列腺内肿瘤的立体定向体放射治疗:hypo-FLAME 试验的 5 年疗效和毒性。
IF 4.9
1区 医学
Q1 ONCOLOGY
引用次数: 0
摘要
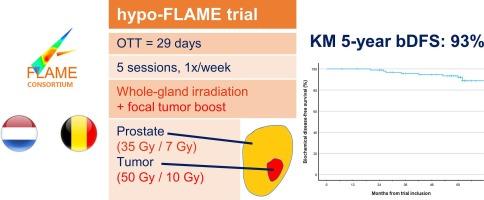
背景:在传统的分次放疗中,对前列腺内病灶增加综合病灶增强与中高危前列腺癌(PCa)患者生化无病生存期(bDFS)的改善有关。此外,全腺立体定向体放疗(SBRT)在治疗低危和中危前列腺癌方面的疗效并不亚于传统放疗。为了研究超低分量前列腺SBRT与等毒灶增强治疗中、高危PCa的组合,我们进行了hypo-FLAME试验:中、高危PCa患者被纳入hypo-FLAME II期试验。所有患者都接受了每周5次、每次35 Gy的全前列腺分次治疗,并对多参数磁共振成像确定的肿瘤进行等效毒性综合增强,最高可达50 Gy。如果超出了正常组织的剂量限制,则会优先于病灶增强剂量。目前的分析报告了5年bDFS、晚期毒性和健康相关生活质量(HRQoL):2016年至2018年间,100名男性接受了治疗,中位随访时间为61个月。估计5年bDFS(95% CI)为93%(86%至97%)。5年后,2+级泌尿生殖系统和胃肠道毒性发生率分别为12%和4%:结论:对中高危PCa患者进行5年随访时,超高分次病灶增强SBRT的生化控制率令人鼓舞。此外,采用等毒性病灶增强的前列腺 SBRT 技术的晚期泌尿生殖系统和胃肠道毒性率也是可以接受的。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Stereotactic body radiotherapy with a focal boost to the intraprostatic tumor for intermediate and high risk prostate cancer: 5-year efficacy and toxicity in the hypo-FLAME trial
Background
The addition of an integrated focal boost to the intraprostatic lesion is associated with improved biochemical disease-free survival (bDFS) in patients with intermediate- and high-risk prostate cancer (PCa) in conventionally fractionated radiotherapy. Furthermore, whole gland stereotactic body radiotherapy (SBRT) demonstrated to be non-inferior to conventional radiotherapy for low- and intermediate-risk PCa. To investigate the combination of ultra-hypofractionated prostate SBRT with iso-toxic focal boosting for intermediate- and high-risk PCa, we performed the hypo-FLAME trial.
Methods
Patients with intermediate- or high-risk PCa were enrolled in the phase II hypo-FLAME trial. All patients were treated with 35 Gy in 5 weekly fractions to the whole prostate gland with an iso-toxic integrated boost up to 50 Gy to the multiparametric MRI-defined tumor(s). If the dose constraints to the normal tissues would be exceeded, these were prioritised over the focal boost dose. The current analysis reports on the 5-year bDFS, late toxicity and health-related quality of life (HRQoL).
Results
Between 2016 and 2018, 100 men were treated with a median follow-up of 61 months. The estimated 5-year bDFS (95 % CI) was 93 % (86 % to 97 %). At 5 years, the prevalence of grade 2 + genitourinary and gastrointestinal toxicity was 12 % and 4 %, respectively.
Conclusion
Ultra-hypofractionated focal boost SBRT is associated with encouraging biochemical control rates up to 5-year follow-up in patients with intermediate- and high-risk PCa. Furthermore, prostate SBRT with iso-toxic focal boosting is associated with acceptable late genitourinary and gastrointestinal toxicity rates.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Radiotherapy and Oncology
医学-核医学
CiteScore
10.30
自引率
10.50%
发文量
2445
审稿时长
45 days
期刊介绍:
Radiotherapy and Oncology publishes papers describing original research as well as review articles. It covers areas of interest relating to radiation oncology. This includes: clinical radiotherapy, combined modality treatment, translational studies, epidemiological outcomes, imaging, dosimetry, and radiation therapy planning, experimental work in radiobiology, chemobiology, hyperthermia and tumour biology, as well as data science in radiation oncology and physics aspects relevant to oncology.Papers on more general aspects of interest to the radiation oncologist including chemotherapy, surgery and immunology are also published.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


