癌细胞膜伪装姜黄素纳米粒子可触发铁蛋白沉积,实现胃癌精准治疗
IF 4.4
2区 医学
Q1 PHARMACOLOGY & PHARMACY
European Journal of Pharmaceutics and Biopharmaceutics
Pub Date : 2024-10-02
DOI:10.1016/j.ejpb.2024.114509
引用次数: 0
摘要
姜黄素(CUR)是一种疏水性多酚,具有相当高的抗肿瘤效率,但由于其在水溶液中溶解性差、稳定性低,且在体内缺乏靶向性,因此临床应用受到限制。在此,我们通过在介孔二氧化硅 NPs(MSN-CUR@CM)上载入 CUR 并包覆同源癌细胞膜(CM),制备了一种肿瘤靶向给药系统。表征分析表明,尺寸约为 70 nm 的 MSN-CUR@CM 具有很高的水溶性和生物相容性。此外,由于MSN-CUR@CM具有细胞自我识别能力,因此在体外和体内均表现出肿瘤靶向性和良好的抗胃癌效果。在已建立的异种移植肿瘤裸鼠模型中,给药后 72 小时,MSN-CUR@CM 在肿瘤部位仍有明显的药物蓄积。此外,MSN-CUR@CM 组的平均肿瘤体积和重量分别是 CUR 组的 3.97 倍和 7.47 倍。MSN-CUR@CM引发了铁凋亡,这是一种伴随着铁依赖性脂质过氧化的非凋亡调节性细胞死亡。进一步的分析表明,MSN-CUR@CUR可上调血红素加氧酶(HO)-1的水平,而下调谷胱甘肽过氧化物酶4(GPX4)在体外SGC7901细胞中的表达,这表明MSN-CUR@CM调节了典型和非典型的铁凋亡途径。总之,我们的研究表明,MSN-CUR@CM 具有高水溶性、生物相容性和肿瘤靶向性,可通过触发铁跃迁在体外和体内抑制胃癌,并提供令人钦佩的癌症治疗效果。本文章由计算机程序翻译,如有差异,请以英文原文为准。
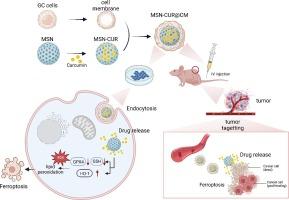
Cancer cell membrane-camouflaged curcumin nanoparticles trigger ferroptosis for accurate gastric cancer therapy
Curcumin (CUR) is a hydrophobic polyphenol with considerable antitumor efficiency, but its clinical application is limited because of its poor solubility and low stability in aqueous solution and lack of targeting in vivo. Herein, we fabricated a tumor-targeting drug delivery system by loading CUR and cloaking homologous cancer cell membrane (CM) onto mesoporous silica NPs (MSN-CUR@CM). Characterization analysis showed that MSN-CUR@CM with a size of approximately 70 nm showed high water solubility and biocompatibility. Besides, MSN-CUR@CM exhibited tumor-targeting and excellent anti-gastric cancer efficiency both in vitro and in vivo owing to the cellular self-recognition of CM. In the established xenograft tumor nude mouse model, it was still significantly drug accumulated at the tumor site 72 h post administration. In addition, the mean tumor volume and weight of the MSN-CUR@CM group were was 3.97 and 7.47 times smaller than those of the CUR group. Ferroptosis, a type of non-apoptotic regulated cell death accompanied by iron-dependent lipid peroxidation, was triggered by MSN-CUR@CM. Further analysis demonstrated that MSN-CUR@CUR upregulated heme oxygenase (HO-1) levels whereas it downregulated the expression of glutathione peroxidase 4 (GPX4) in SGC7901 cells in vitro, indicating that the canonical and noncanonical ferroptosis pathways were regulated by MSN-CUR@CM. In conclusion, our study demonstrated that MSN-CUR@CM with high water solubility, biocompatibility, and tumor-targeting properties inhibited gastric cancer both in vitro and in vivo by triggering ferroptosis and provided an admirable cancer therapy efficacy.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
8.80
自引率
4.10%
发文量
211
审稿时长
36 days
期刊介绍:
The European Journal of Pharmaceutics and Biopharmaceutics provides a medium for the publication of novel, innovative and hypothesis-driven research from the areas of Pharmaceutics and Biopharmaceutics.
Topics covered include for example:
Design and development of drug delivery systems for pharmaceuticals and biopharmaceuticals (small molecules, proteins, nucleic acids)
Aspects of manufacturing process design
Biomedical aspects of drug product design
Strategies and formulations for controlled drug transport across biological barriers
Physicochemical aspects of drug product development
Novel excipients for drug product design
Drug delivery and controlled release systems for systemic and local applications
Nanomaterials for therapeutic and diagnostic purposes
Advanced therapy medicinal products
Medical devices supporting a distinct pharmacological effect.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


