钴催化的丙二腈对映体选择性硼氢化还原脱对称反应
IF 19.2
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
人们广泛利用丙二腈的高含氮量和多种反应活性来获得富氮精细化学品。虽然丙二腈的简单取代可以产生结构多样的季碳,但它们与对映体丰富的分子,尤其是生物活性化合物中普遍存在的手性胺的接触仍然罕见。在此,我们报告了一种由钴催化的二取代丙二腈不对称还原反应,从而得到高度官能化的β-季胺。钴盐和硼氢化钠配对生成钴酸酐中间体并启动还原反应。同时,二腈的对映体控制是通过量身定制的双噁唑啉配体来实现的,该配体有两个大的侧翼,形成了一个狭窄的间隙来容纳旁生的腈,从而限制了络合物的 C(ipso)-C(α) 键旋转。结合季碳上所有取代基的广泛衍生可能性,这种不对称还原释放了从丙二腈作为大宗化学原料到复杂的手性含氮分子的途径。本文章由计算机程序翻译,如有差异,请以英文原文为准。
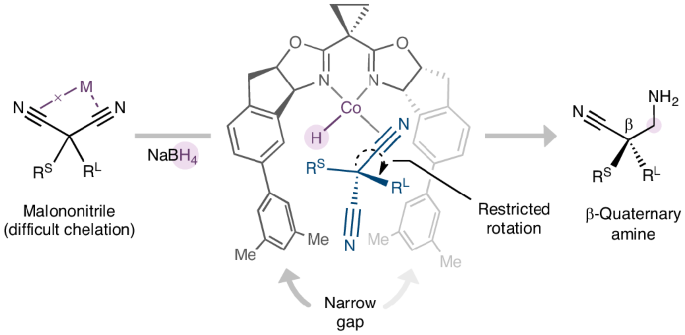
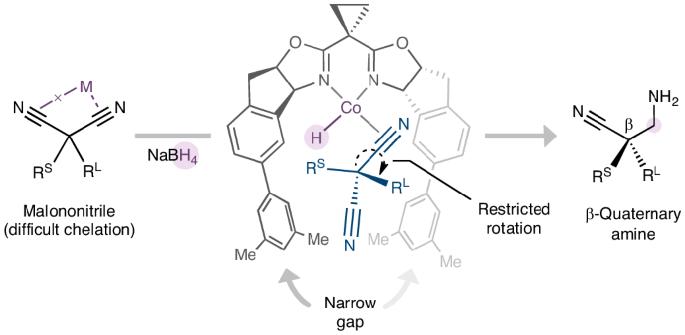
Cobalt-catalysed desymmetrization of malononitriles via enantioselective borohydride reduction
The high nitrogen content and diverse reactivity of malononitrile are widely harnessed to access nitrogen-rich fine chemicals. Although the facile substitutions of malononitrile can give structurally diverse quaternary carbons, their access to enantioenriched molecules, particularly chiral amines that are prevalent in bioactive compounds, remains rare. Here we report a cobalt-catalysed desymmetric reduction of disubstituted malononitriles to give highly functionalized β-quaternary amines. The pair of cobalt salt and sodium borohydride is proposed to generate a cobalt-hydride intermediate and initiate the reduction. Meanwhile, the enantiocontrol of the dinitrile is achieved through a tailored bisoxazoline ligand with two large flanks that create a narrow gap to host the bystanding nitrile and thus restrict the C(ipso)−C(α) bond rotation of the complexed one. Combined with the extensive derivatization possibilities of all substituents on the quaternary carbon, this asymmetric reduction unlocks pathways from malononitrile as a bulk chemical feedstock to intricate, chiral nitrogen-containing molecules. Malononitriles are widely used precursors for the synthesis of diverse enantioenriched nitrogen-containing molecules, but controlling the stereochemistry of their asymmetric transformations is challenging. Now, the desymmetric reduction of disubstituted malononitriles to chiral amines has been achieved, enabled by a bidentate ligand with extended flanks that can differentiate between the precursor’s nitrile groups through tailored steric pairings.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature chemistry
化学-化学综合
CiteScore
29.60
自引率
1.40%
发文量
226
审稿时长
1.7 months
期刊介绍:
Nature Chemistry is a monthly journal that publishes groundbreaking and significant research in all areas of chemistry. It covers traditional subjects such as analytical, inorganic, organic, and physical chemistry, as well as a wide range of other topics including catalysis, computational and theoretical chemistry, and environmental chemistry.
The journal also features interdisciplinary research at the interface of chemistry with biology, materials science, nanotechnology, and physics. Manuscripts detailing such multidisciplinary work are encouraged, as long as the central theme pertains to chemistry.
Aside from primary research, Nature Chemistry publishes review articles, news and views, research highlights from other journals, commentaries, book reviews, correspondence, and analysis of the broader chemical landscape. It also addresses crucial issues related to education, funding, policy, intellectual property, and the societal impact of chemistry.
Nature Chemistry is dedicated to ensuring the highest standards of original research through a fair and rigorous review process. It offers authors maximum visibility for their papers, access to a broad readership, exceptional copy editing and production standards, rapid publication, and independence from academic societies and other vested interests.
Overall, Nature Chemistry aims to be the authoritative voice of the global chemical community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


