物种特征、景观质量以及与蜜蜂的花卉资源重叠决定了病毒在植物授粉者网络中的传播方式
IF 13.9
1区 生物学
Q1 ECOLOGY
引用次数: 0
摘要
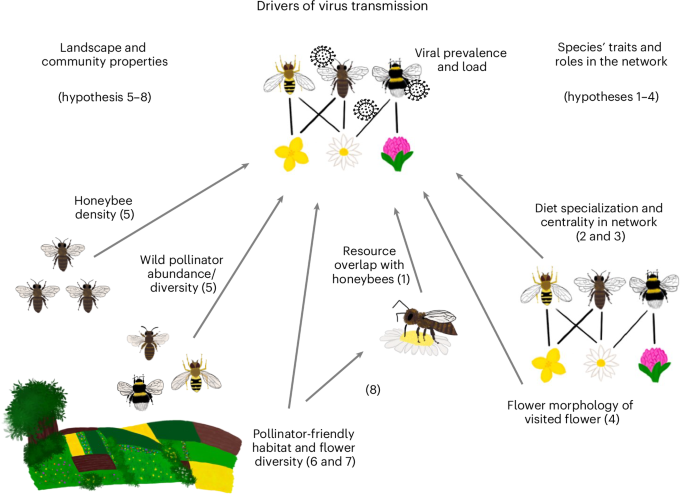
新出现的传染性疾病对传粉昆虫构成威胁。人为的土地利用变化以及随之而来的植物与传粉昆虫之间相互作用的改变,可能会加强病毒通过花朵在传粉昆虫之间的传播。在这里,我们研究了物种的特征、在花朵-访问网络中的作用以及景观尺度因素如何在12个不同的授粉者友好(花朵丰富)生境景观中驱动19种野生蜜蜂和食蚜蝇物种感染关键蜜蜂病毒--黑蜂王细胞病毒(BQCV)和畸形翅病毒。管理蜜蜂的病毒负荷量平均比野生授粉昆虫高十倍以上。当花卉资源的使用与蜜蜂重叠时,野生传粉昆虫的病毒量更高,这表明蜜蜂是病毒库宿主,病毒量随传粉昆虫的数量和蜜蜂的病毒量增加而增加。病毒流行率随着景观中有利于传粉昆虫的栖息地数量的增加而降低,部分原因是与蜜蜂的花卉资源重叠减少。黑蜂后细胞病毒载量随着野生授粉者在网络中的中心地位和访问的碟形花比例的增加而减少。我们的研究结果突显了与蜜蜂的资源重叠、物种特征和在花卉访问网络中的作用以及花卉丰富的授粉者栖息地在影响病毒传播方面的复杂相互作用。本文章由计算机程序翻译,如有差异,请以英文原文为准。

Species traits, landscape quality and floral resource overlap with honeybees determine virus transmission in plant–pollinator networks
Emerging infectious diseases pose a threat to pollinators. Virus transmission among pollinators via flowers may be reinforced by anthropogenic land-use change and concomitant alteration of plant–pollinator interactions. Here, we examine how species’ traits and roles in flower-visitation networks and landscape-scale factors drive key honeybee viruses—black queen cell virus (BQCV) and deformed wing virus—in 19 wild bee and hoverfly species, across 12 landscapes varying in pollinator-friendly (flower-rich) habitat. Viral loads were on average more than ten times higher in managed honeybees than in wild pollinators. Viral loads in wild pollinators were higher when floral resource use overlapped with honeybees, suggesting these as reservoir hosts, and increased with pollinator abundance and viral loads in honeybees. Viral prevalence decreased with the amount of pollinator-friendly habitat in a landscape, which was partly driven by reduced floral resource overlap with honeybees. Black queen cell virus loads decreased with a wild pollinator’s centrality in the network and the proportion of visited dish-shaped flowers. Our findings highlight the complex interplay of resource overlap with honeybees, species traits and roles in flower-visitation networks and flower-rich pollinator habitat shaping virus transmission. Human-driven landscape change may alter disease transmission among insect pollinators. Here, the authors show that species traits, flower-rich habitat and floral resource overlap with honeybees explain load and prevalence of viruses in wild bees and hoverflies co-occurring with honeybees.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature ecology & evolution
Agricultural and Biological Sciences-Ecology, Evolution, Behavior and Systematics
CiteScore
22.20
自引率
2.40%
发文量
282
期刊介绍:
Nature Ecology & Evolution is interested in the full spectrum of ecological and evolutionary biology, encompassing approaches at the molecular, organismal, population, community and ecosystem levels, as well as relevant parts of the social sciences. Nature Ecology & Evolution provides a place where all researchers and policymakers interested in all aspects of life's diversity can come together to learn about the most accomplished and significant advances in the field and to discuss topical issues. An online-only monthly journal, our broad scope ensures that the research published reaches the widest possible audience of scientists.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


