免疫代谢线索对植入生物材料周围的微环境进行重组和重编程
IF 26.8
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
摘要
循环中的单核细胞在植入的生物材料周围组织和其他发炎组织中浸润并协调免疫反应。在这里,我们展示了生物材料微环境中的免疫代谢线索以一种依赖于趋化因子受体 2(CCR2)和 C-X3-C motif 趋化因子受体 1(CX3CR1)的方式控制着包括中性粒细胞和单核细胞在内的免疫细胞的迁移。这会影响巨噬细胞和树突状细胞群的组成和活化状态,最终协调促炎性、暂时性和抗炎性 CCR2+、CX3CR1+ 和 CCR2+ CX3CR1+ 免疫细胞群的相对组成。在无定形聚乳酸植入物中,通过抑制糖酵解来改变免疫代谢,从而主要通过髓系细胞来推动有利于再生的微环境。在结晶聚乳酸植入物中,T辅助2细胞和产生白细胞介素-4的γδ+ T细胞与表达精氨酸酶-1的髓系细胞一起,为塑造代谢重编程的促再生微环境做出了重大贡献。我们的研究结果证明了一个前提,即局部代谢状态可调节生物材料微环境中的炎症过程。本文章由计算机程序翻译,如有差异,请以英文原文为准。

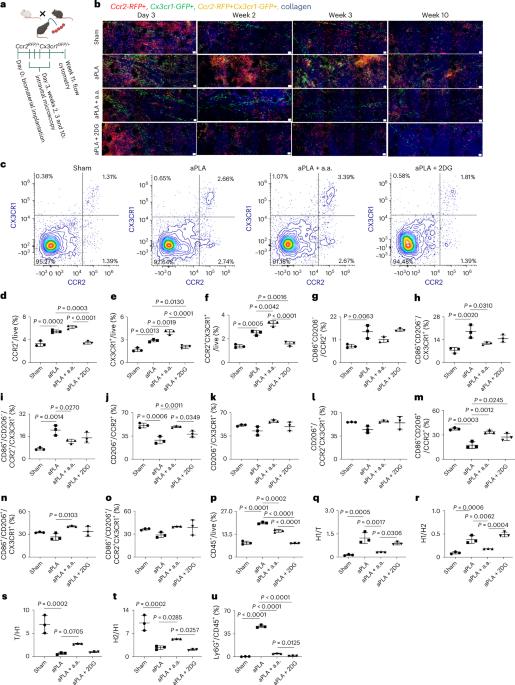
Immunometabolic cues recompose and reprogram the microenvironment around implanted biomaterials
Circulating monocytes infiltrate and coordinate immune responses in tissues surrounding implanted biomaterials and in other inflamed tissues. Here we show that immunometabolic cues in the biomaterial microenvironment govern the trafficking of immune cells, including neutrophils and monocytes, in a manner dependent on the chemokine receptor 2 (CCR2) and the C-X3-C motif chemokine receptor 1 (CX3CR1). This affects the composition and activation states of macrophage and dendritic cell populations, ultimately orchestrating the relative composition of pro-inflammatory, transitory and anti-inflammatory CCR2+, CX3CR1+ and CCR2+ CX3CR1+ immune cell populations. In amorphous polylactide implants, modifying immunometabolism by glycolytic inhibition drives a pro-regenerative microenvironment principally by myeloid cells. In crystalline polylactide implants, together with arginase-1-expressing myeloid cells, T helper 2 cells and γδ+ T cells producing interleukin-4 substantially contribute to shaping the metabolically reprogrammed pro-regenerative microenvironment. Our findings inform the premise that local metabolic states regulate inflammatory processes in the biomaterial microenvironment. Immunometabolic cues surrounding implanted biomaterials govern the trafficking of subsets of neutrophils, monocytes and other immune cells, and determine the relative composition of pro-inflammatory and anti-inflammatory immune cell populations.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Biomedical Engineering
Medicine-Medicine (miscellaneous)
CiteScore
45.30
自引率
1.10%
发文量
138
期刊介绍:
Nature Biomedical Engineering is an online-only monthly journal that was launched in January 2017. It aims to publish original research, reviews, and commentary focusing on applied biomedicine and health technology. The journal targets a diverse audience, including life scientists who are involved in developing experimental or computational systems and methods to enhance our understanding of human physiology. It also covers biomedical researchers and engineers who are engaged in designing or optimizing therapies, assays, devices, or procedures for diagnosing or treating diseases. Additionally, clinicians, who make use of research outputs to evaluate patient health or administer therapy in various clinical settings and healthcare contexts, are also part of the target audience.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


