在 COVID-19 后出现持续呼吸道症状和放射学异常的患者中,肺泡巨噬细胞源性增殖性单核细胞扩增
IF 27.7
1区 医学
Q1 IMMUNOLOGY
引用次数: 0
摘要
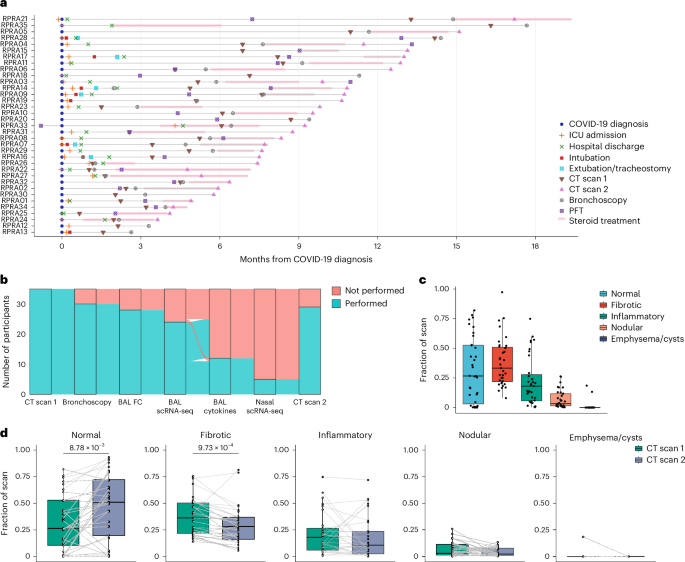
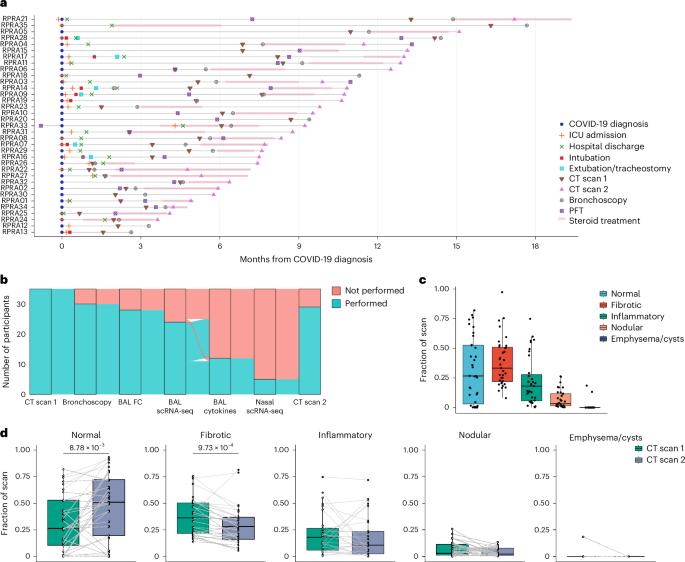
在小鼠模型中,单核细胞衍生的肺泡巨噬细胞驱动肺损伤和肺纤维化,并与人类的肺纤维化有关。有人认为,单核细胞衍生的肺泡巨噬细胞会形成一种表型,在损伤消退时促进肺修复。我们比较了 35 名 COVID-19 急性后遗症患者肺泡空间的单细胞和细胞因子图谱,这些患者有持续性呼吸道症状,胸部计算机断层扫描显示异常,随后病情有所改善或进展。支气管肺泡灌洗液中单核细胞衍生的肺泡巨噬细胞的数量、基因表达程序和单核细胞趋化因子 CCL2 的水平与放射学纤维化的严重程度呈正相关。纤维化缓解期或进展期患者的单核细胞衍生肺泡巨噬细胞表达了相同的一组促纤维化基因。我们的研究结果表明,单核细胞衍生的肺泡巨噬细胞并不具有独特的修复表型,这突显了它们作为肺修复失败的生物标志物和潜在治疗靶点的作用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Profibrotic monocyte-derived alveolar macrophages are expanded in patients with persistent respiratory symptoms and radiographic abnormalities after COVID-19
Monocyte-derived alveolar macrophages drive lung injury and fibrosis in murine models and are associated with pulmonary fibrosis in humans. Monocyte-derived alveolar macrophages have been suggested to develop a phenotype that promotes lung repair as injury resolves. We compared single-cell and cytokine profiling of the alveolar space in a cohort of 35 patients with post-acute sequelae of COVID-19 who had persistent respiratory symptoms and abnormalities on a computed tomography scan of the chest that subsequently improved or progressed. The abundance of monocyte-derived alveolar macrophages, their gene expression programs, and the level of the monocyte chemokine CCL2 in bronchoalveolar lavage fluid positively associated with the severity of radiographic fibrosis. Monocyte-derived alveolar macrophages from patients with resolving or progressive fibrosis expressed the same set of profibrotic genes. Our findings argue against a distinct reparative phenotype in monocyte-derived alveolar macrophages, highlighting their utility as a biomarker of failed lung repair and a potential target for therapy. Misharin, Sala and colleagues show that in patients with lung fibrosis after COVID-19, monocyte-derived alveolar macrophages activate an inflammatory and fibrotic program that was similar in patients with either resolving or progressing fibrosis.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Immunology
医学-免疫学
CiteScore
40.00
自引率
2.30%
发文量
248
审稿时长
4-8 weeks
期刊介绍:
Nature Immunology is a monthly journal that publishes the highest quality research in all areas of immunology. The editorial decisions are made by a team of full-time professional editors. The journal prioritizes work that provides translational and/or fundamental insight into the workings of the immune system. It covers a wide range of topics including innate immunity and inflammation, development, immune receptors, signaling and apoptosis, antigen presentation, gene regulation and recombination, cellular and systemic immunity, vaccines, immune tolerance, autoimmunity, tumor immunology, and microbial immunopathology. In addition to publishing significant original research, Nature Immunology also includes comments, News and Views, research highlights, matters arising from readers, and reviews of the literature. The journal serves as a major conduit of top-quality information for the immunology community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


