ARF1 区室通过成熟为循环内体引导货物流动
IF 17.3
1区 生物学
Q1 CELL BIOLOGY
引用次数: 0
摘要
细胞膜的平衡是通过内吞途径和分泌途径的细胞器之间严格调控的膜和货物流动来维持的。被小 GTP 酶 ARF1 募集到膜上的适配蛋白复合物(APs)促进了货物的选择和纳入贩运中间体。根据经典模型,小囊泡可促进高尔基体、内体和质膜之间的双向长程运输。在这里,我们结合使用 CRISPR-Cas9 基因编辑、活细胞高时间(快速共聚焦)或空间(刺激发射耗竭)显微镜以及相关的光镜和电子显微镜,重新审视了囊泡运输机制的细胞内组织。我们描述了容纳凝集素和不同AP的管泡ARF1区室的特征。我们的发现揭示了两类功能不同的 ARF1 区室,每一类都由不同的 APs 组合装饰。核周ARF1区室有助于高尔基体输出分泌货物,而外周ARF1区室则参与早期内体下游的内细胞再循环。与经典的长程囊泡穿梭模型相反,我们观察到 ARF1 区室脱落 ARF1 并成熟为再循环内体。在缺乏 AP-1 的情况下,这一成熟过程会受损,并导致贩运缺陷。总之,这些数据强调了 ARF1 区室在后高尔基体分拣中的关键作用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
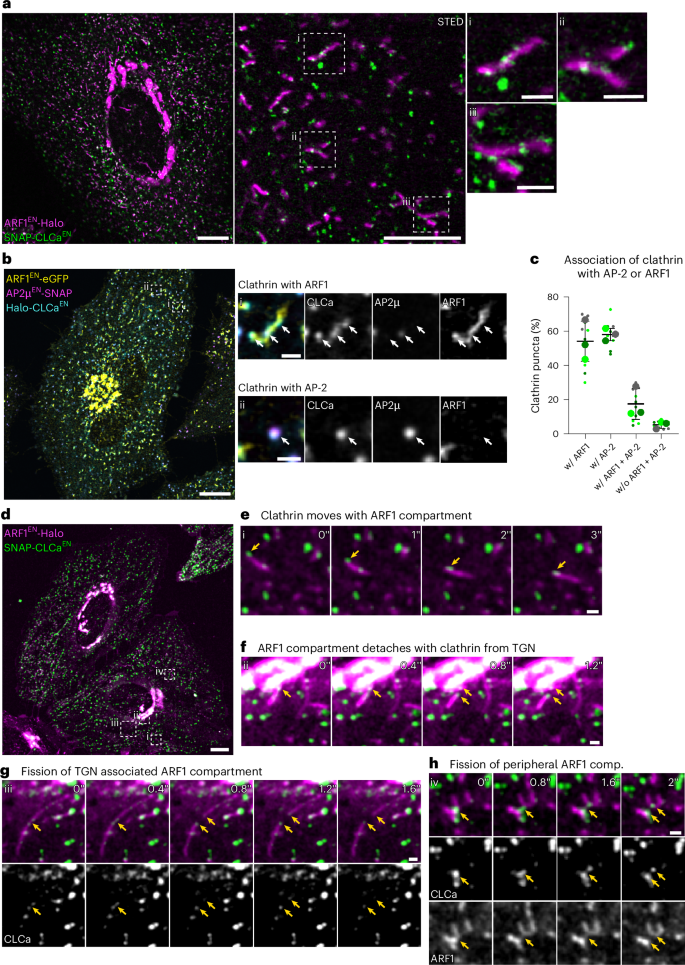
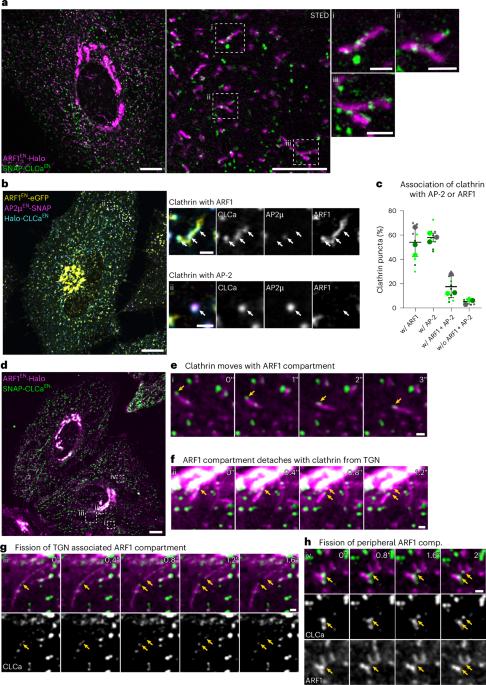
ARF1 compartments direct cargo flow via maturation into recycling endosomes
Cellular membrane homoeostasis is maintained via a tightly regulated membrane and cargo flow between organelles of the endocytic and secretory pathways. Adaptor protein complexes (APs), which are recruited to membranes by the small GTPase ARF1, facilitate cargo selection and incorporation into trafficking intermediates. According to the classical model, small vesicles would facilitate bi-directional long-range transport between the Golgi, endosomes and plasma membrane. Here we revisit the intracellular organization of the vesicular transport machinery using a combination of CRISPR-Cas9 gene editing, live-cell high temporal (fast confocal) or spatial (stimulated emission depletion) microscopy as well as correlative light and electron microscopy. We characterize tubulo-vesicular ARF1 compartments that harbour clathrin and different APs. Our findings reveal two functionally different classes of ARF1 compartments, each decorated by a different combination of APs. Perinuclear ARF1 compartments facilitate Golgi export of secretory cargo, while peripheral ARF1 compartments are involved in endocytic recycling downstream of early endosomes. Contrary to the classical model of long-range vesicle shuttling, we observe that ARF1 compartments shed ARF1 and mature into recycling endosomes. This maturation process is impaired in the absence of AP-1 and results in trafficking defects. Collectively, these data highlight a crucial role for ARF1 compartments in post-Golgi sorting. Stockhammer, Adarska et al. describe ARF1 compartments as the site of adaptor- and clathrin-dependent post-Golgi sorting. Shedding of ARF1 and maturation into recycling endosomes drives sorting of secretory and endocytic recycling cargo.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Cell Biology
生物-细胞生物学
CiteScore
28.40
自引率
0.90%
发文量
219
审稿时长
3 months
期刊介绍:
Nature Cell Biology, a prestigious journal, upholds a commitment to publishing papers of the highest quality across all areas of cell biology, with a particular focus on elucidating mechanisms underlying fundamental cell biological processes. The journal's broad scope encompasses various areas of interest, including but not limited to:
-Autophagy
-Cancer biology
-Cell adhesion and migration
-Cell cycle and growth
-Cell death
-Chromatin and epigenetics
-Cytoskeletal dynamics
-Developmental biology
-DNA replication and repair
-Mechanisms of human disease
-Mechanobiology
-Membrane traffic and dynamics
-Metabolism
-Nuclear organization and dynamics
-Organelle biology
-Proteolysis and quality control
-RNA biology
-Signal transduction
-Stem cell biology
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


