Li3Sb7Se12:具有超低导热性的帕伏尼石同系物
IF 7
2区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
含锂半导体可应用于固态电池、热电和中子探测器。Li3Sb7Se12 是一种新的三元半导体,与在单斜空间群 C2/m 中结晶的矿物本杰明石(Ag3Sb7S12)结构相同。本杰明石属于普长石矿物家族,通式为 Mn+1(Bi/Sb)2Qn+5 (n = 0-8),其中 n 是由共面八面体组成的八面体构件长度。Li3Sb7Se12 属于 n = 7,它与苯并氨石的不同之处在于 Li+ 和 Sb3+ 原子间的占位紊乱。在 300-873 K 的温度范围内,它具有 0.53-0.37 W m-1 K-1 的超低晶格热导率,是一种 p 型半导体。利用 DFT 计算进行的化学键分析表明,锑的分层键和畸变配位环境在其热传输特性中发挥了突出作用,从而导致了超低的晶格热导率,而硒则在带隙附近的电子结构中占主导地位。本文章由计算机程序翻译,如有差异,请以英文原文为准。
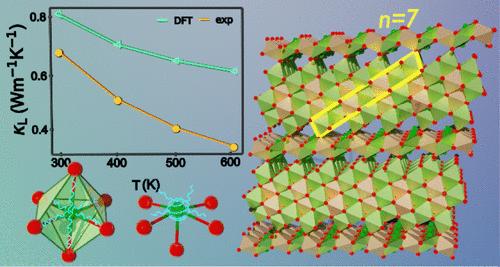
Li3Sb7Se12: A Pavonite Homologue with Ultralow Thermal Conductivity
Lithium-containing semiconductors have applications in solid-state batteries, thermoelectrics, and neutron detectors. Li3Sb7Se12 is a new ternary semiconductor isostructural to the mineral benjaminite (Ag3Sb7S12) crystallizing in monoclinic space group C2/m. Benjaminite belongs to the pavonite mineral family with the general formula Mn+1(Bi/Sb)2Qn+5 (n = 0–8), where n is the octahedral building block length consisting of face-sharing octahedra. Li3Sb7Se12 belongs to n = 7, and it differs from benjaminite by exhibiting occupancy disorder between Li+ and Sb3+ atoms. It exhibits exceptionally low lattice thermal conductivities of 0.53–0.37 W m–1 K–1 in the temperature range 300–873 K and is a p-type semiconductor. Chemical bonding analysis using DFT calculations indicates that hierarchical bonding and the distorted coordination environment of antimony play a prominent role in its heat transport properties, leading to ultralow lattice thermal conductivity, while selenium dominates the electronic structure near the bandgap.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemistry of Materials
工程技术-材料科学:综合
CiteScore
14.10
自引率
5.80%
发文量
929
审稿时长
1.5 months
期刊介绍:
The journal Chemistry of Materials focuses on publishing original research at the intersection of materials science and chemistry. The studies published in the journal involve chemistry as a prominent component and explore topics such as the design, synthesis, characterization, processing, understanding, and application of functional or potentially functional materials. The journal covers various areas of interest, including inorganic and organic solid-state chemistry, nanomaterials, biomaterials, thin films and polymers, and composite/hybrid materials. The journal particularly seeks papers that highlight the creation or development of innovative materials with novel optical, electrical, magnetic, catalytic, or mechanical properties. It is essential that manuscripts on these topics have a primary focus on the chemistry of materials and represent a significant advancement compared to prior research. Before external reviews are sought, submitted manuscripts undergo a review process by a minimum of two editors to ensure their appropriateness for the journal and the presence of sufficient evidence of a significant advance that will be of broad interest to the materials chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


