元基因组分析显示氢基膜生物膜反应器中存在大量混养菌、异养菌和同源乙酸菌
IF 11.4
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
摘要
在富氢脱硝系统中经常能观察到异养微生物,并推测它们有助于提高系统性能。然而,它们在氢基膜生物膜反应器(H2-MBfR)系统中的作用和代谢途径仍不清楚。本研究的目的是阐明驱动异养反硝化的基本机制。为此,对反硝化性能较高的 H2-MBfR 进行了元基因组分析,重点研究微生物群落的代谢功能。与 H2 氧化、有机碳代谢和反硝化有关的功能基因是研究的主要目标。这项分析发现了大量与生物膜中有机碳化合物氧化有关的基因,表明生物膜具有异养反硝化的潜力。对元基因组组装基因组(MAGs)中相关基因的调查表明,主要是混养或异养生物,而不是强制性自养生物。值得注意的是,展示出最高丰度相关基因的 MAGs 都隶属于 Hydrogenophaga 和 Thauera,这意味着它们作为利用 H2 和有机底物的混养生物,在反硝化 H2-MBfR 方面发挥着重要作用。鉴定出 11 个 MAG,推测它们来自同源醋酸菌,这表明醋酸可能有助于异养菌的增殖。根据这些元基因组研究结果,确定了可能的代谢途径,以解释 H2-MBfR 生物膜中的异养反硝化作用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
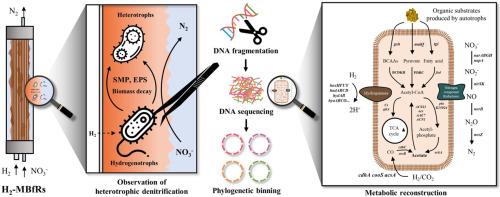
Metagenomic analysis reveals abundance of mixotrophic, heterotrophic, and homoacetogenic bacteria in a hydrogen-based membrane biofilm reactor
Heterotrophic microorganisms are frequently observed in hydrogenotrophic denitrification systems and are presumed to contribute to their improved performance. However, their roles and metabolic pathways in the hydrogen-based membrane biofilm reactor (H2-MBfR) system remain unclear. The objective of this study was to elucidate the underlying mechanisms driving heterotrophic denitrification. For this purpose, metagenomic analysis was conducted on an H2-MBfR showing higher denitrification performance, focusing on the metabolic function of the microbial community. Functional genes related to H2 oxidation, organic carbon metabolism, and denitrification were the major targets of interest. This analysis revealed a substantial number of genes associated with the oxidation of organic carbon compounds in the biofilm, suggesting its potential for heterotrophic denitrification. Investigation of the genes of interest in metagenome-assembled genomes (MAGs) has demonstrated a predominance of mixotrophs or heterotrophs rather than obligate autotrophs. Notably, MAGs exhibiting the highest abundance of genes of interest were affiliated with Hydrogenophaga and Thauera, implying their significant role in denitrifying the H2-MBfR as mixotrophs utilizing both H2 and organic substrates. The identification of 11 MAGs, presumed to originate from homoacetogens suggested that acetate might contribute to the proliferation of heterotrophs. Based on these metagenomic findings, possible metabolic pathways were identified to explain heterotrophic denitrification within the H2-MBfR biofilms.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Water Research
环境科学-工程:环境
CiteScore
20.80
自引率
9.40%
发文量
1307
审稿时长
38 days
期刊介绍:
Water Research, along with its open access companion journal Water Research X, serves as a platform for publishing original research papers covering various aspects of the science and technology related to the anthropogenic water cycle, water quality, and its management worldwide. The audience targeted by the journal comprises biologists, chemical engineers, chemists, civil engineers, environmental engineers, limnologists, and microbiologists. The scope of the journal include:
•Treatment processes for water and wastewaters (municipal, agricultural, industrial, and on-site treatment), including resource recovery and residuals management;
•Urban hydrology including sewer systems, stormwater management, and green infrastructure;
•Drinking water treatment and distribution;
•Potable and non-potable water reuse;
•Sanitation, public health, and risk assessment;
•Anaerobic digestion, solid and hazardous waste management, including source characterization and the effects and control of leachates and gaseous emissions;
•Contaminants (chemical, microbial, anthropogenic particles such as nanoparticles or microplastics) and related water quality sensing, monitoring, fate, and assessment;
•Anthropogenic impacts on inland, tidal, coastal and urban waters, focusing on surface and ground waters, and point and non-point sources of pollution;
•Environmental restoration, linked to surface water, groundwater and groundwater remediation;
•Analysis of the interfaces between sediments and water, and between water and atmosphere, focusing specifically on anthropogenic impacts;
•Mathematical modelling, systems analysis, machine learning, and beneficial use of big data related to the anthropogenic water cycle;
•Socio-economic, policy, and regulations studies.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


