对 Ni/CeO2 催化剂的详细表征研究发现,镍的可用性是影响二氧化碳甲烷化性能的主要因素
IF 6.5
1区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
催化剂的结构对其性能有很大影响。在此,我们研究了 Ni/CeO2 的物理化学特性,试图得出二氧化碳甲烷化的结构-活性关系。结合多种表征方法(X 射线衍射 (XRD)、4D 扫描透射电子显微镜 (4D-STEM)、CO 化学吸附和储氧能力研究等),我们清楚地证明了在 CO2 甲烷化过程中,Ni/CeO2 中 Ni 粒径、Ni 可用性(即可催化的 Ni)和 Ni-CeO2 界面氧空位的影响。其中强调了暴露的镍活性位点的作用,并提出了两种可能的优化方案,即改变支撑物的煅烧温度和最终煅烧气氛,以获得镍 NPs 在 CeO2 上更好的分散。这两种改性方法都不会影响反应路线,Ni/CeO2 的活性差异可以用甲酸盐物种的不同氢化率来解释,这一点已被原位扩散反射红外傅立叶变换光谱(DRIFTS)测量所证实。本文章由计算机程序翻译,如有差异,请以英文原文为准。
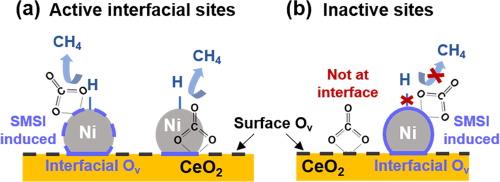
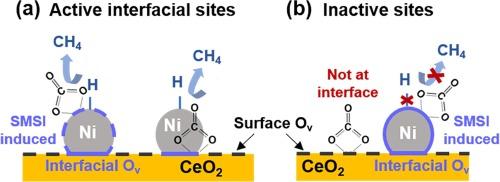
A detailed characterization study of Ni/CeO2 catalysts identifies Ni availability as the primary factor affecting CO2 methanation performance
The structure of a catalyst has a strong impact on its performance. Here we investigate the physicochemical properties of Ni/CeO2 in an attempt to draw structure–activity relationships for CO2 methanation. A combination of characterisation methods (X-ray diffraction (XRD), 4D Scanning Transmission Electron Microscopy (4D-STEM), CO chemisorption and oxygen storage capacity study etc.) clearly demonstrates the effect of Ni crystallite size, Ni availability (i.e. catalytically accessible Ni) and oxygen vacancies at the Ni-CeO2 interface in Ni/CeO2 during CO2 methanation. Among them, the role of exposed Ni active sites is highlighted, and two possible optimisation schemes i.e. changing the support calcination temperature and the final calcination atmosphere are proposed to obtain a better dispersal of Ni NPs (nanoparticles) on CeO2. Both modification methods do not affect the reaction route, and the activity differences of Ni/CeO2 can be explained by the various hydrogenation rate of formate species, as confirmed by in situ diffuse-reflectance infrared Fourier-transform spectroscopy (DRIFTS) measurements.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Catalysis
工程技术-工程:化工
CiteScore
12.30
自引率
5.50%
发文量
447
审稿时长
31 days
期刊介绍:
The Journal of Catalysis publishes scholarly articles on both heterogeneous and homogeneous catalysis, covering a wide range of chemical transformations. These include various types of catalysis, such as those mediated by photons, plasmons, and electrons. The focus of the studies is to understand the relationship between catalytic function and the underlying chemical properties of surfaces and metal complexes.
The articles in the journal offer innovative concepts and explore the synthesis and kinetics of inorganic solids and homogeneous complexes. Furthermore, they discuss spectroscopic techniques for characterizing catalysts, investigate the interaction of probes and reacting species with catalysts, and employ theoretical methods.
The research presented in the journal should have direct relevance to the field of catalytic processes, addressing either fundamental aspects or applications of catalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


