钯催化末端炔烃与硝基烯烃的支氢化氨基羰基化反应
IF 6.5
1区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
我们报告了一种创新的钯催化加氢氨基羰基化方法,它能有效地将硝基烯烃和末端炔烃转化为 α、β-不饱和酰胺。这种方法具有广泛的底物兼容性,产率为 32% 至 87%,并具有极佳的区域选择性。双吡啶配体和水杨酸是该反应有效性的核心,它们在优化选择性和产物产率方面发挥着关键作用。机理实验显示,芳胺是整个反应的关键中间体,突出了催化循环中的独特途径。与传统程序相比,该方案采用了多种硝基烯烃和 Mo(CO)6 作为直接氮源和固体羰基源,从而简化了工艺流程,并通过减少对有害 CO 气体的依赖提高了安全性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
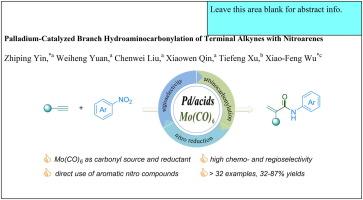
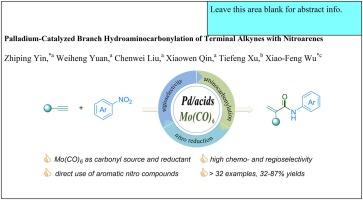
Palladium-Catalyzed branch hydroaminocarbonylation of terminal alkynes with nitroarenes
We report an innovative palladium-catalyzed hydroaminocarbonylation approach that efficiently converts nitroarenes and terminal alkynes into α,β-unsaturated amides. This method, with broad substrate compatibility, delivers 32% to 87% yields with excellent regioselectivity. Central to the effectiveness of this reaction are the bipyridine ligand and salicylic acid, which play pivotal roles in optimizing both selectivity and product yield. Mechanistic experiments revealed that arylamine serves as a critical intermediate in the overall reaction, highlighting a unique pathway in the catalytic cycle. In contrast to conventional procedures, this protocol employs diverse nitroarenes and Mo(CO)6 as direct nitrogen and solid carbonyl sources, streamlining the process and enhancing safety by reducing the reliance on hazardous CO gas.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Catalysis
工程技术-工程:化工
CiteScore
12.30
自引率
5.50%
发文量
447
审稿时长
31 days
期刊介绍:
The Journal of Catalysis publishes scholarly articles on both heterogeneous and homogeneous catalysis, covering a wide range of chemical transformations. These include various types of catalysis, such as those mediated by photons, plasmons, and electrons. The focus of the studies is to understand the relationship between catalytic function and the underlying chemical properties of surfaces and metal complexes.
The articles in the journal offer innovative concepts and explore the synthesis and kinetics of inorganic solids and homogeneous complexes. Furthermore, they discuss spectroscopic techniques for characterizing catalysts, investigate the interaction of probes and reacting species with catalysts, and employ theoretical methods.
The research presented in the journal should have direct relevance to the field of catalytic processes, addressing either fundamental aspects or applications of catalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


