溶质不会结晶!相图揭开结晶 "魔法 "的神秘面纱
IF 17.5
1区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
摘要
晶体在自然界和技术领域无处不在。尽管了解结晶机制非常重要,但传统理论已被证明是不够的。对相图的研究表明,这些理论的缺陷源于热力学上无效的假设,包括溶质是结晶相以及 Ksp 描述了溶解度。热力学要求营养物(溶剂)是结晶相。这一观点提供了严格描述相图特征的工具,以明确确定结晶系统的组分以及稀释剂对营养液液相的影响。研究表明,稀释剂可以改变营养物质的液相,但并不参与晶体生长的速率决定步骤。研究发现,决定晶体生长速率的步骤是晶体相界通过类似熔体的中间体的传播。这一过渡区理论模型准确地描述了所有浓度-温度条件下的晶体生长速率,并解决了晶体形态和生长速率的 "谜题"。本文章由计算机程序翻译,如有差异,请以英文原文为准。
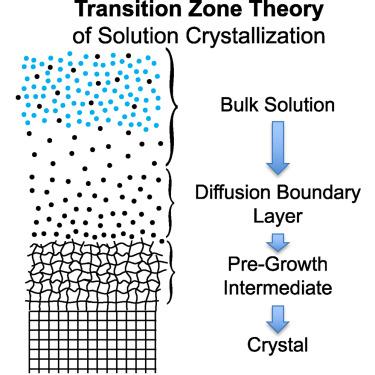
Solutes don’t crystallize! Insights from phase diagrams demystify the “magic” of crystallization
Crystals are ubiquitous in nature and technology. Despite the importance of understanding mechanisms of crystallization, conventional theories have proved inadequate. Consideration of phase diagrams reveals that these theories’ failings result from thermodynamically invalid assumptions, including that solutes are the crystallizing phase and that Ksp describes solubility. Thermodynamics requires that the nutrient (solvent) be the crystallizing phase. This perspective provides tools to rigorously characterize a phase diagram to explicitly determine the components of the crystallizing system and the influence of the diluent on the liquidus of the nutrient. It is shown that diluents can change the liquidus of the nutrient but do not participate in the rate-determining step of crystal growth. The rate-determining step is found to be the propagation of the crystalline phase boundary through a melt-like intermediate. This transition-zone theory model accurately describes crystal growth rates for all concentration-temperature conditions and resolves “riddles” of crystal morphology and growth rates.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Matter
MATERIALS SCIENCE, MULTIDISCIPLINARY-
CiteScore
26.30
自引率
2.60%
发文量
367
期刊介绍:
Matter, a monthly journal affiliated with Cell, spans the broad field of materials science from nano to macro levels,covering fundamentals to applications. Embracing groundbreaking technologies,it includes full-length research articles,reviews, perspectives,previews, opinions, personnel stories, and general editorial content.
Matter aims to be the primary resource for researchers in academia and industry, inspiring the next generation of materials scientists.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


