反应中间产物对单原子催化剂的动态活化:Rh/Fe3O4(001) 上甲酸的转化
IF 11.3
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
支撑单原子催化剂(SAC)的稳定性和活性是限制我们探索和利用其催化特性的关键但又相互对立的因素。本研究展示了一种催化剂的工作原理,这种催化剂在存在表面中间产物的情况下被动态激活,并在反应完成后恢复到稳定但无活性的形式。我们采用原子定义的 Rh/Fe3O4(001)催化剂,展示了结构稳定的 Rh 与表面八面体铁位点结合后,如何脱稳形成高活性的 Rh 金刚原子和小团簇。甲酸的转化最初会产生表面甲酸盐和羟基物种,我们将此作为模型反应来探究此类过程的动态。我们发现,表面羟基通过 Mars-van Krevelen 机制重组为水,降低了 Rh 的配位,引发其向活性 Rh 金刚原子的转化。反应完成后(表面-中间体游离催化剂),Rh 金刚原子回到稳定的八面体 Rh 位点,从而限制了 Rh 的烧结。由于在许多酸碱和氧化还原化学反应中都能观察到晶格氧交换,因此该过程可广泛用于控制一系列 SAC 的活化和稳定性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
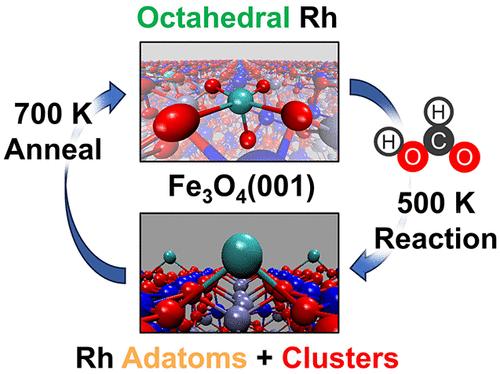
Dynamic Activation of Single-Atom Catalysts by Reaction Intermediates: Conversion of Formic Acid on Rh/Fe3O4(001)
The stability and activity of supported single-atom catalysts (SACs) represent critical yet opposing factors limiting our ability to explore and exploit their catalytic properties. This study demonstrates the operation of a catalyst that is dynamically activated in the presence of surface intermediates and reverts to a stable but inactive form when the reaction is completed. We employ atomically defined Rh/Fe3O4(001) catalysts to demonstrate how structurally stable Rh, bound in surface octahedral Fe sites, gets destabilized to form highly active Rh adatoms and small clusters. Conversion of formic acid, leading initially to surface formate and hydroxyl species, is employed as a model reaction to probe the dynamics of such processes. We find that surface hydroxyl recombination to water through the Mars–van Krevelen mechanism reduces Rh coordination, triggering its conversion to active Rh adatoms. Upon completion of the reaction (surface-intermediate free catalyst), Rh adatoms return back to the stable octahedral Rh sites, limiting Rh sintering. Since lattice oxygen exchange is observed in many acid–base and redox chemistries, the process can be broadly applicable to controlling the activation and stability of the range of SACs.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


