高性能氧化镁吸附剂对硝酸盐和亚硝酸盐阴离子协同促进机制的新解释
IF 8.1
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
摘要
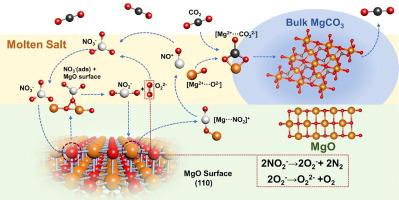
关于硝酸盐和亚硝酸盐阴离子物种协同促进氧化镁捕集二氧化碳的机理研究很少,至今仍未完全弄清。本文系统研究了熔盐促进氧化镁吸附剂的二氧化碳吸附性能,深入揭示了硝酸盐和亚硝酸盐协同促进机制。以NaNO2/KNO3为促进剂,合成了一系列氧化镁基固体吸附剂,并将其应用于二氧化碳捕集。硝酸盐和亚硝酸盐混合促进剂的加入大大提高了氧化镁的吸附能力,在最佳条件下二氧化碳捕获量可达 10.25 mmol-g-1。纳米片状氧化镁吸附剂具有更快的吸附动力学和更好的稳定性,并提出了硝酸盐和亚硝酸盐修饰氧化镁的可能反应机理。首先,NO3- 与氧化镁的晶格氧(O12-)相互作用生成 NO2- 和 O22-。其次,Mg2+ 与游离的 NO2- 相互作用形成 [Mg---NO2]+,并进一步克服能障生成 [Mg2+---O2-] 离子对。O22- 并不稳定,容易发生副反应。但是,NO2- 可以转化为 O2-,O2- 又进一步生成 O22-,从而促进了 NO3- 的再生。熔盐的引入可显著降低反应能垒,生成[Mg2+--O2-]离子对所需的能垒从 6.86 eV 降至 3.50 eV。吸附动力学研究表明,纳米片状氧化镁有利于与促进剂充分接触,在表面反应控制阶段具有更快的反应动力学。这些关于硝酸盐和亚硝酸盐阴离子协同促进机制的见解有望为进一步设计基于氧化镁的高性能二氧化碳吸附剂提供指导。本文章由计算机程序翻译,如有差异,请以英文原文为准。
A new explanation for the synergistic promoted mechanism of nitrate and nitrite anion species for the high performance MgO adsorbent
The mechanism of CO2 capture on the nitrate and nitrite anion species synergistic promoted MgO has been rarely studied and still not fully understood. Herein we report a systematic investigation on the CO2 adsorption performance of molten salts-promoted MgO adsorbent to deeply reveal the synergistic promoted mechanism of nitrate and nitrite. A series of MgO-based solid adsorbents were synthesized and applied for CO2 capture using NaNO2/KNO3 as promoters. The addition of mixed nitrate and nitrite promoters greatly enhances the adsorption capacity of MgO, exhibiting CO2 capture up to 10.25 mmol·g−1 under optimal conditions. The MgO-based adsorbent in the form of nanosheets has faster adsorption kinetics and better stability, and the possible reaction mechanism of MgO modified by nitrate and nitrite was proposed. Firstly, NO3– interacts with the lattice oxygen (O12-) of MgO to form NO2– and O22–. Secondly, Mg2+ interacts with the free NO2– to form [Mg···NO2]+ and further overcomes the energy barrier to generate [Mg2+···O2–] ion pairs. O22– is not stable and prone to side reaction. However, NO2– can be converted into O2–, and O2– is further generated to O22–, facilitating regeneration of NO3–. The introduction of molten salt can significantly reduce the reaction energy barrier, and the energy barrier required to generate [Mg2+···O2–] ion pairs is reduced from 6.86 eV to 3.50 eV. Adsorption kinetics studies reveal that the nano-flake MgO facilitates the full contact with the promoter and has faster reaction kinetics in the surface reaction control stage. These insights into the synergistic promoted mechanism of nitrate and nitrite anion species is expected to provide guidance for the further design of the high performance MgO-based CO2 adsorbent.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Separation and Purification Technology
工程技术-工程:化工
CiteScore
14.00
自引率
12.80%
发文量
2347
审稿时长
43 days
期刊介绍:
Separation and Purification Technology is a premier journal committed to sharing innovative methods for separation and purification in chemical and environmental engineering, encompassing both homogeneous solutions and heterogeneous mixtures. Our scope includes the separation and/or purification of liquids, vapors, and gases, as well as carbon capture and separation techniques. However, it's important to note that methods solely intended for analytical purposes are not within the scope of the journal. Additionally, disciplines such as soil science, polymer science, and metallurgy fall outside the purview of Separation and Purification Technology. Join us in advancing the field of separation and purification methods for sustainable solutions in chemical and environmental engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


