烷基胺的有机催化不对称 α-C-H 功能化
IF 42.8
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
对广泛存在的非手性烷基胺进行催化对映选择性 α-C-H 功能化,可为手性胺的合成提供一种理想的方法。然而,由于烷基胺的α-C-H 具有惰性,因此将其活化为催化不对称反应的阴离子亲核物是一项尚未解决的重要挑战。在此,我们介绍了如何将 N-芳基亚甲基保护的烷基胺活化为不对称共轭加成和曼尼希反应的碳阴离子。这些成果代表了合成手性 α,α-二烷基胺的一种有趣且普遍有用的方法。更重要的是,它们凸显了 N-芳基亚胺保护胺作为手性胺不对称合成的现成且广泛应用的合成物所具有的巨大潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
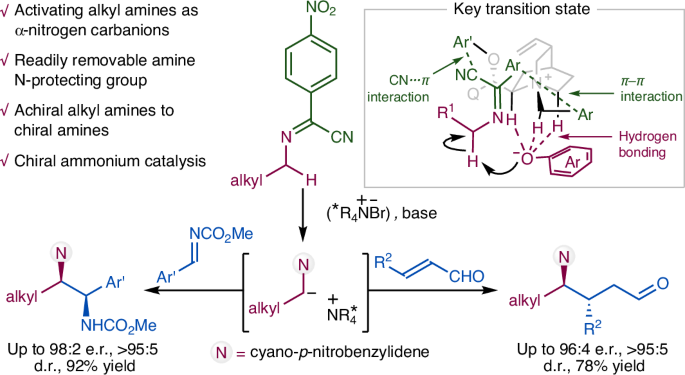
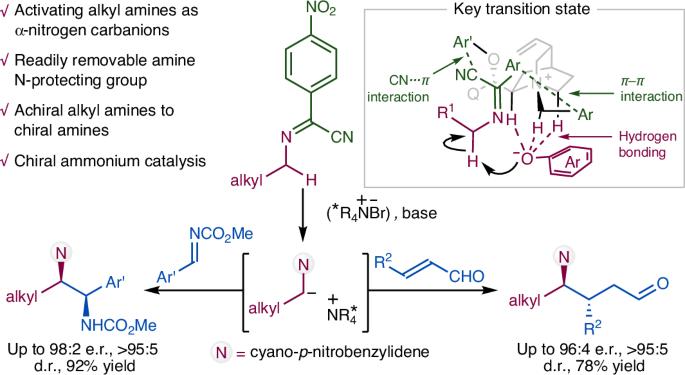
Organocatalytic asymmetric α-C–H functionalization of alkyl amines
Catalytic enantioselective α-C–H functionalization of widely available achiral alkyl amines could provide an ideal synthetic approach towards chiral amines. However, the inert nature of the α-C–H of alkyl amines renders their activation as carbanionic nucleophiles for catalytic asymmetric reactions an important yet unmet challenge. Here we describe how N-arylidene-protected alkyl amines could be activated as carbanions for asymmetric conjugate addition and the Mannich reaction. These results represent an intriguing and generally useful approach to the synthesis of chiral α,α-dialkyl amines. More importantly, they highlight the enormous potential of N-arylidene-protected amines as readily available and widely applicable synthons for the asymmetric synthesis of chiral amines. The catalytic activation of alkyl amines as α-nitrogen carbanions is challenging. Now the activation of N-arylidene-protected alkyl amines as carbanions by chiral ammonium organocatalysis for asymmetric conjugate addition and the Mannich reaction is reported, affording chiral α,α-dialkyl amines.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Catalysis
Chemical Engineering-Bioengineering
CiteScore
52.10
自引率
1.10%
发文量
140
期刊介绍:
Nature Catalysis serves as a platform for researchers across chemistry and related fields, focusing on homogeneous catalysis, heterogeneous catalysis, and biocatalysts, encompassing both fundamental and applied studies. With a particular emphasis on advancing sustainable industries and processes, the journal provides comprehensive coverage of catalysis research, appealing to scientists, engineers, and researchers in academia and industry.
Maintaining the high standards of the Nature brand, Nature Catalysis boasts a dedicated team of professional editors, rigorous peer-review processes, and swift publication times, ensuring editorial independence and quality. The journal publishes work spanning heterogeneous catalysis, homogeneous catalysis, and biocatalysis, covering areas such as catalytic synthesis, mechanisms, characterization, computational studies, nanoparticle catalysis, electrocatalysis, photocatalysis, environmental catalysis, asymmetric catalysis, and various forms of organocatalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


