从海洋药用褐藻中提取的化学成分 Scytalidium lignicola SC228。
IF 3.2
2区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
本研究分离并鉴定了一种海洋药用褐藻马尾藻(Sargassum cristaefolium)衍生真菌菌株Scytalidium lignicola SC228。研究人员对该真菌菌株液体发酵产物的提取物进行了柱层析,最终纯化出 8 种化合物。通过光谱分析确定了它们的结构特征,并通过单 X 射线衍射分析和改进的莫舍尔法进一步确定了它们的绝对构型,即四种以前未曾描述过的化合物,即鞘氨醇 A-C (1-3)和 (3S,4S)-5-氯-3,4-二氢-4,6,8-三羟基-3-甲基-1H-2-苯并吡喃-1-酮(4),以及四种已知化合物 5-8。所有分离物都进行了抗炎和抗血管生成试验。与姜黄素(IC50 = 2.7 ± 0.3 μM)相比,化合物 1-4、7 和 8 在脂多糖激活的 BV-2 微神经胶质细胞中显示出中等程度的一氧化氮生成抑制活性,IC50 在 19.6 ± 0.1 到 49.0 ± 1.2 μM之间。与索拉非尼(IC50 = 5.50 ± 1.50 μM)相比,化合物 5-7 对 EPCs 的生长具有中度到强效的抑制作用,IC50 在 0.5 ± 0.1 到 42.7 ± 0.9 μM之间。本文章由计算机程序翻译,如有差异,请以英文原文为准。
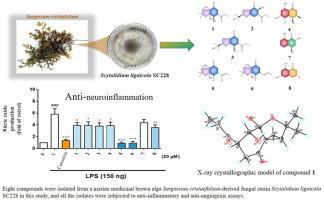
Chemical constituents from marine medicinal brown alga-derived Scytalidium lignicola SC228
In this study, a marine medicinal brown alga Sargassum cristaefolium-derived fungal strain Scytalidium lignicola SC228 has been isolated and identified. Column chromatography of the extracts from liquid-fermented products of the fungal strain was carried out, and led to the purification of eight compounds. Their structures were characterized by spectroscopic analysis, and the absolute configurations were further established by single X-ray diffraction analysis and modified Mosher's method as four previously undescribed compounds, namely scytabenzofurans A–C (1–3), and (3S,4S)-5-chloro-3,4-dihydro-4,6,8-trihydroxy-3-methyl-1H-2-benzopyran-1-one (4), along with four known compounds 5–8. All the isolates were subjected to anti-inflammatory and anti-angiogenic assays. Compounds 1–4, 7, and 8 showed moderate nitric oxide production inhibitory activities in lipopolysaccharide-activated BV-2 microglial cells with IC50 in the range of 19.6 ± 0.1 to 49.0 ± 1.2 μM in comparison with that of curcumin (IC50 = 2.7 ± 0.3 μM). Compounds 5–7 exhibited moderate to potent inhibitory effects on EPCs growth with IC50 in the range of 0.5 ± 0.1 to 42.7 ± 0.9 μM as compared to sorafenib (IC50 = 5.50 ± 1.50 μM).
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Phytochemistry
生物-植物科学
CiteScore
6.40
自引率
7.90%
发文量
443
审稿时长
39 days
期刊介绍:
Phytochemistry is a leading international journal publishing studies of plant chemistry, biochemistry, molecular biology and genetics, structure and bioactivities of phytochemicals, including ''-omics'' and bioinformatics/computational biology approaches. Phytochemistry is a primary source for papers dealing with phytochemicals, especially reports concerning their biosynthesis, regulation, and biological properties both in planta and as bioactive principles. Articles are published online as soon as possible as Articles-in-Press and in 12 volumes per year. Occasional topic-focussed special issues are published composed of papers from invited authors.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


