L 型配体上取代基的共振效应介导了对金纳米簇前沿轨道能量的合成控制
IF 4.6
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
金属纳米簇的配体可用于控制其特性和反应性,但指导其使用的框架仍未确定。对带有对位和偏甲基及-甲氧基的 Au8(PPh3)72+ 和 Au9(PPh3)83+ 纳米簇的 Hammett 研究表明,共振效应(而非感应效应)产生了涉及簇核局部轨道的 HOMO-LUMO 转换的定量偏移。单个配体交换显示,这些位移是由七个配体中的四个引起的,与感应效应不一致。量子化学计算没有预测到金原子电荷随哈米特参数变化的趋势,但预测了包括金原子在内的共振结构的键长趋势。计算出的轨道显示特定的对位 OMe 氧孤对电子对 HOMO 的贡献,表明从核心到特定配体的脱位。这些结果表明,可以绘制出包括金和配体在内的共振结构,为调节纳米簇电子结构和能量传递提供指导。本文章由计算机程序翻译,如有差异,请以英文原文为准。
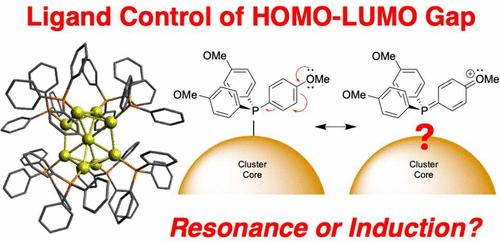
Resonance Effects from Substituents on L-Type Ligands Mediate Synthetic Control of Gold Nanocluster Frontier Orbital Energies
The ligands of metal nanoclusters can be used to control their properties and reactivity, but a framework guiding their use remains elusive. Hammett studies of Au8(PPh3)72+ and Au9(PPh3)83+ nanoclusters with para- and meta-methyl and -methoxy groups indicate that resonance effects, not inductive effects, yield quantitative shifts of the HOMO–LUMO transitions involving orbitals local to the cluster core. Individual ligand exchanges reveal that these shifts are caused by only four of seven ligands, inconsistent with inductive effects. Quantum chemical calculations predict no trend in Au atom charges with respect to Hammett parameter but do predict bond length trends expected for a resonance structure that includes the Au atoms. Computed orbitals show contributions from specific para-OMe oxygen lone pairs to the HOMO, indicating delocalization from the core to specific ligands. These results suggest that resonance structures could be drawn including Au and ligands, guiding efforts to modulate nanocluster electronic structure and energy transfer.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

The Journal of Physical Chemistry Letters
CHEMISTRY, PHYSICAL-NANOSCIENCE & NANOTECHNOLOGY
CiteScore
9.60
自引率
7.00%
发文量
1519
审稿时长
1.6 months
期刊介绍:
The Journal of Physical Chemistry (JPC) Letters is devoted to reporting new and original experimental and theoretical basic research of interest to physical chemists, biophysical chemists, chemical physicists, physicists, material scientists, and engineers. An important criterion for acceptance is that the paper reports a significant scientific advance and/or physical insight such that rapid publication is essential. Two issues of JPC Letters are published each month.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


