适用于预制备功能性和工程性双聚酰胺的新型胺先策略:糠胺作为双呋喃二胺/二酸单体的唯一呋喃源
IF 4.1
2区 化学
Q2 POLYMER SCIENCE
引用次数: 0
摘要
生物质基聚酰胺(bioPAs)是一种可再生材料,是工程领域石油基聚酰胺的可行替代品。然而,人们对设计具有可调特性的功能性生物聚酰胺的关注还很有限。本文以糠胺为唯一的呋喃源,首先利用胺酸氧化包覆法直接从双呋喃二胺单体制备双呋喃二酸单体(胺先法),这与基于蓖麻油的酸先法制备 PA1010 的策略完全不同。然后,将制备的多功能二胺/二酸单体进行缩聚反应,得到带有或不带有功能性悬垂基团的全呋喃基生物 PA。通过设计二胺单体上两个呋喃环之间的间隔结构,可以在更大范围内调节生物 PA 的性能,包括玻璃化转变温度、降解特性、溶解性等。通过后聚合改性,进一步开发了带有阳离子或阴离子基团的带电荷生物PA。这些带相反电荷的生物PA 具有相同的主链结构,但不同的下垂基团,由于其刚性主链而形成多孔聚电解质复合物。因此,这项研究为制备功能性和工程性呋喃基生物 PAs 提供了一种新策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
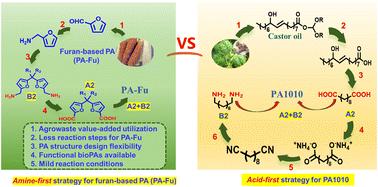
A novel amine-first strategy suitable for preparing both functional and engineering bio-polyamides: furfurylamine as the sole furan source for bisfuranic diamine/diacid monomers†
Biomass-based polyamides (bioPAs) are renewable materials that are viable alternatives to petroleum-based polyamides in the engineering field. However, limited attention has been paid to designing functional bioPAs with tunable properties. Herein, by taking furfurylamine as the sole furan source, we first utilized amine-acid oxidative conversion to prepare a bisfuranic diacid monomer directly from a bisfuranic diamine monomer (amine-first strategy), and it was totally different from the castor oil-based acid-first strategy for preparing PA1010. Then the as-prepared multifunctional diamine/diacid monomers underwent polycondensation to obtain all-furan-based bioPAs with or without functional pendant groups. The properties of bioPAs, including glass transition temperature, degradation character, solubility, etc., can be regulated over a larger range through the design of the spacer structure between two furan rings on diamine monomers. Charged bioPAs with cationic or anionic groups were further developed by postpolymerization modification. The oppositely charged bioPAs, sharing an identical main chain structure but different pendant groups, formed porous polyelectrolyte complexes owing to their rigid main chain. Therefore, this research provides a novel strategy for preparing both functional and engineering furan-based bioPAs.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Polymer Chemistry
POLYMER SCIENCE-
CiteScore
8.60
自引率
8.70%
发文量
535
审稿时长
1.7 months
期刊介绍:
Polymer Chemistry welcomes submissions in all areas of polymer science that have a strong focus on macromolecular chemistry. Manuscripts may cover a broad range of fields, yet no direct application focus is required.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


