在无溶剂介质中捕获二氧化碳并将其转化为双环碳酸酯的绿色化学酶法
IF 7.3
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
一种在无溶剂条件下将二氧化碳、缩水甘油和有机酐捕获并通过化学酶转化为双(环碳酸酯)的可持续方法已经得到证实。该化学酶法工艺基于两个连续的催化步骤,可以通过分离操作执行,也可以在单锅组合系统内执行,利用了离子液体(IL)技术和生物催化剂结合产生的协同效应。第一步,在温和的反应条件下(70 °C,6 小时),脂肪酶催化不同二酰供体(如戊二酸酐、琥珀酸酐、丁二酸二甲酯等)与缩水甘油在无溶剂条件下发生酯化反应,生成相应的二缩水甘油酯衍生物,收率高达 41%。第二步,在共价连接的 1-癸基-2-甲基咪唑鎓 IL(类支撑离子液体相,SILLP)的催化下,在无溶剂条件下,二氧化碳(来自废气源,二氧化碳纯度为 15%)与这些二缩水甘油酯发生环加成反应,合成双(环碳酸酯),在 45 °C 和 1 兆帕二氧化碳压力下反应 8 小时后,产率高达 65%。反应系统的两个关键元素(生物催化剂和 SILLP)都被成功回收并重复使用了至少 5 个操作周期。最后,我们采用了不同的指标来评估本文所报道的双(环碳酸酯)酯无溶剂化学酶法合成的绿色程度。本文章由计算机程序翻译,如有差异,请以英文原文为准。
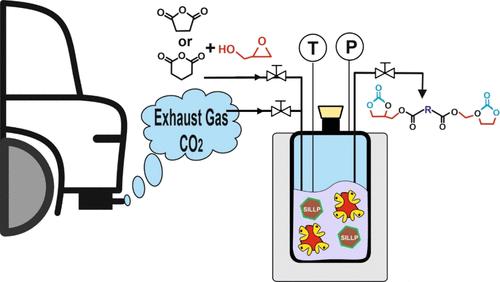
A Green Chemo-Enzymatic Approach for CO2 Capture and Transformation into Bis(cyclic carbonate) Esters in Solvent-Free Media
A sustainable approach for CO2 capture and chemo-enzymatic transformation into bis(cyclic carbonate) esters from CO2, glycidol, and organic anhydrides under solvent-free conditions has been demonstrated. The chemo-enzymatic process is based on two consecutive catalytic steps, which can be executed through separated operations or within a one-pot combo system, taking advantage of the synergic effects that emerge from integrating ionic liquid (IL) technologies and biocatalysts. In a first step, lipase-catalyzed transesterification and esterification reactions of different diacyl donors (e.g., glutaric anhydride, succinic anhydride, dimethyl succinate, etc.) with glycidol in solvent-free under mild reaction conditions (70 °C, 6 h) produce the corresponding diglycidyl ester derivatives in up to 41% yield. By a second step, the synthesis of bis(cyclic carbonate) esters was carried out as a result of the cycloaddition reaction of CO2 (from an exhausted gas source, 15% CO2 purity) on these diglycidyl esters, catalyzed by the covalently attached 1-decyl-2-methylimidazolium IL (supported ionic liquid-like phase, SILLP), in solvent-free condition, leading up to 65% yield after 8 h at 45 °C and 1 MPa CO2 pressure. Both key elements of the reaction system (biocatalyst and SILLP) were successfully recovered and reused for at least 5 operational cycles. Finally, different metrics have been applied to assess the greenness of the solvent-free chemo-enzymatic synthesis of bis(cyclic carbonate) esters here reported.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Sustainable Chemistry & Engineering
CHEMISTRY, MULTIDISCIPLINARY-ENGINEERING, CHEMICAL
CiteScore
13.80
自引率
4.80%
发文量
1470
审稿时长
1.7 months
期刊介绍:
ACS Sustainable Chemistry & Engineering is a prestigious weekly peer-reviewed scientific journal published by the American Chemical Society. Dedicated to advancing the principles of green chemistry and green engineering, it covers a wide array of research topics including green chemistry, green engineering, biomass, alternative energy, and life cycle assessment.
The journal welcomes submissions in various formats, including Letters, Articles, Features, and Perspectives (Reviews), that address the challenges of sustainability in the chemical enterprise and contribute to the advancement of sustainable practices. Join us in shaping the future of sustainable chemistry and engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


