磷酸化-缩合级联用于 C 核苷的生物催化合成
IF 11.5
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
C 核苷是合成抗感染药物和治疗性核酸的重要目标。在这里,我们展示了由 d-核糖(Rib)和尿嘧啶产生假尿嘧啶 5′-磷酸(Ψ5P)的磷酸化-缩合级联反应。Rib(∼1.0 M)在乙酰磷酸水溶液(1.15 M)中通过 ATP(5 mM)被偶联激酶在 O5 处磷酸化。利用Ψ5P C-糖苷酶,Rib5P 中间体(收率≥90%)与摩尔当量的尿嘧啶(以固体形式提供)反应,定量得到Ψ5P。对试剂组成、自动 pH 值控制和固液传质进行优化的单锅反应,从 10 毫升体积中产生了 2.2 克 Ψ5P(产率:38 克/升/小时)。级联反应的合成灵活性体现在其他戊糖(d-阿拉伯糖、2-脱氧-Rib、d-木糖)和尿嘧啶类似物(6-氨基、2-硫代、4-硫代)上。总之,我们展示了戊糖磷酸化以及 C-C 键形成缩合步骤的大规模强化(≥50 倍),这些步骤是高效合成 C 核苷(单磷酸)的整个多酶级联转化过程。本文章由计算机程序翻译,如有差异,请以英文原文为准。
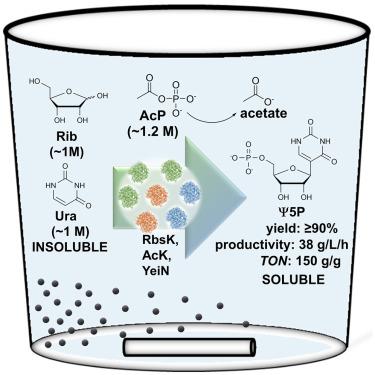
Phosphorylation-condensation cascade for biocatalytic synthesis of C-nucleosides
C-nucleosides are important targets for synthesis as anti-infective agents and building blocks for therapeutic nucleic acids. Here, we show phosphorylation-condensation cascade reaction to produce pseudouridine 5′-phosphate (Ψ5P) from d-ribose (Rib) and uracil. Rib (∼1.0 M) was phosphorylated at O5 in aqueous acetyl phosphate (1.15 M) via ATP (5 mM) by coupled kinases. Using Ψ5P C-glycosidase, Rib5P intermediate (≥90% yield) was reacted with the mole equivalent of uracil, supplied as a solid, to give Ψ5P in quantitative yield. One-pot reaction optimized for reagent composition, automated pH control, and solid-liquid mass transfer yielded ∼2.2 g Ψ5P (productivity: 38 g/L/h) from 10-mL volume. Synthetic flexibility of the cascade reaction was shown with other pentoses (d-arabinose, 2-deoxy-Rib, d-xylose) and analogs of uracil (6-amino, 2-thio, 4-thio). Collectively, we show massive intensification (≥50-fold) of the pentose phosphorylation as well as the C–C bond-forming condensation step of the overall multienzyme cascade transformation for efficient C-nucleoside (monophosphate) synthesis.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
10.50
自引率
6.40%
发文量
0
期刊介绍:
Chem Catalysis is a monthly journal that publishes innovative research on fundamental and applied catalysis, providing a platform for researchers across chemistry, chemical engineering, and related fields. It serves as a premier resource for scientists and engineers in academia and industry, covering heterogeneous, homogeneous, and biocatalysis. Emphasizing transformative methods and technologies, the journal aims to advance understanding, introduce novel catalysts, and connect fundamental insights to real-world applications for societal benefit.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


