利用基于四苯基丁-1,3-二烯的 AIE 活性结构单元的噻唑噻唑连接固态发射线性和多孔有机聚合物
IF 3.9
2区 化学
Q2 POLYMER SCIENCE
引用次数: 0
摘要
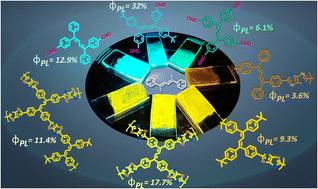
构建具有聚集诱导发射(AIE)活性结构单元的线性或多孔有机聚合物(POP)已经引起了足够的重视,因为它成功地表现出了传统发光体通常不具备的固态发射特性。在此,我们采用无金属一步合成法,利用三种新设计和合成的含四苯基丁-1,3-二烯(TPB)的醛 (-CHO) AIEgens 构建了一系列噻唑噻唑连接的线性和支链多孔有机聚合物 (POP)。通过 1H、13C 和 13C MAS NMR、FTIR 和 MALDI-ToF 研究,对新合成的单体、线性聚合物和 POPs 进行了全面表征。在 THF:水混合物中研究了单体和线性聚合物的 AIE 特性,而在溶液中则进行了随温度变化的发射研究,以了解不溶性多孔聚合物的 AIE 行为。研究结果表明,线性聚合物和多孔聚合物在激发态的分子动力学特性明显不同。尽管如此,所有新合成的聚合物都表现出固态发射,其中多孔聚合物 POP-1 在黄色区域的发光效果极佳(PLQY = 17.7%)。这一结果首次证明了 TPB 衍生物可以作为固态高发光效率持久性有机污染物的骨架,为光电子学的不同应用创造了巨大的空间。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Thiazolothiazole-linked solid-state emissive linear and porous organic polymers utilizing tetraphenyl-buta-1,3-diene-based AIE-active building blocks†
Constructing linear or porous organic polymers with aggregation-induced emission (AIE)-active building blocks has garnered abundant attention owing to their successful manifestation of solid-state emissive properties, which are usually absent in traditional luminophores. Herein, three newly designed and synthesized aldehyde (–CHO)-containing tetraphenyl-buta-1,3-diene (TPB)-based AIEgens have been utilized to construct a series of thiazolothiazole-linked linear polymers and branched porous organic polymers (POPs), following a metal-free one-step synthesis. The newly synthesised monomers, linear polymers and POPs have been characterized thoroughly with 1H, 13C and 13C MAS NMR, FTIR and MALDI-ToF studies. The AIE properties of the monomers and the linear polymers have been studied in a THF : water mixture, whereas temperature-dependent emission studies have been carried out in solution to understand the AIE behaviour of the insoluble porous polymers. The results suggest that the molecular dynamics in the excited states are distinctly different in the linear and the porous polymers. Nevertheless, all the newly synthesized polymers exhibit solid-state emission, with excellent luminescence (PLQY = 17.7%) in the yellow region being obtained for the porous polymer POP-1. This result for the first time demonstrates that TPB derivatives can act as a backbone for POPs with high luminescence efficiency in the solid state, creating immense scope for different applications in optoelectronics.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Polymer Chemistry
POLYMER SCIENCE-
CiteScore
8.60
自引率
8.70%
发文量
535
审稿时长
1.7 months
期刊介绍:
Polymer Chemistry welcomes submissions in all areas of polymer science that have a strong focus on macromolecular chemistry. Manuscripts may cover a broad range of fields, yet no direct application focus is required.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


