GPR34 是 ILC1 介导的抗肿瘤免疫的代谢免疫检查点
IF 27.7
1区 医学
Q1 IMMUNOLOGY
引用次数: 0
摘要
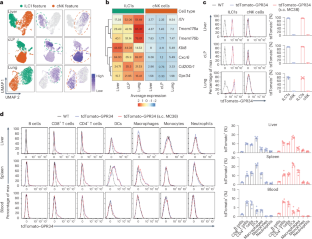
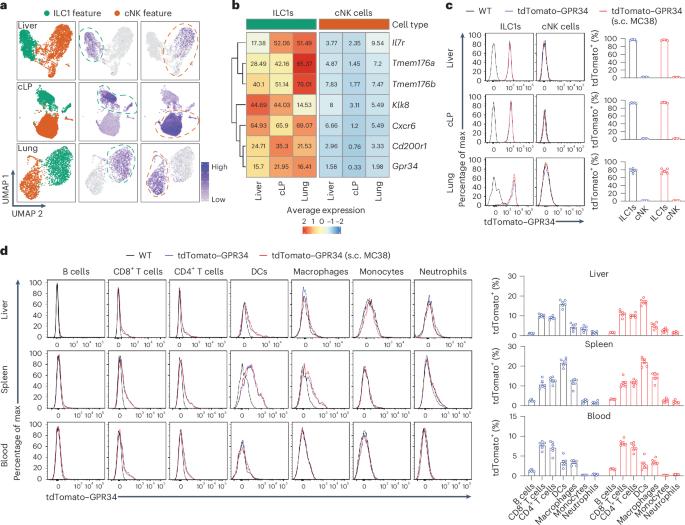
1型先天性淋巴细胞(ILC1s)是一类具有抗肿瘤活性的组织驻留细胞,这表明它可能在实体瘤免疫监视中发挥作用,但目前还不清楚操纵ILC1s是否能诱导有效的抗肿瘤免疫反应。在这里,我们发现溶血磷脂酰丝氨酸(LysoPS)受体 G 蛋白偶联受体 34(GPR34)在肿瘤微环境中的 ILC1s 上高表达,而在传统的自然杀伤细胞上却没有表达。LysoPS在肿瘤微环境中富集,可通过GPR34抑制ILC1的活化。遗传性删除肿瘤中LysoPS合成酶Abhd16a的表达或ILC1s中Gpr34的表达或拮抗GPR34可增强ILC1的抗肿瘤活性。在癌症患者中,肿瘤中 ABHD16A 的表达或 ILC1s 中 GPR34 的表达与 ILC1s 或 ILC1 样细胞的抗肿瘤活性成反比。因此,我们的研究结果表明,操纵 ILC1s 可以诱导有效的抗肿瘤免疫,而 GPR34 是一种代谢免疫检查点,可以作为开发基于 ILC1 的免疫疗法的靶点。本文章由计算机程序翻译,如有差异,请以英文原文为准。
GPR34 is a metabolic immune checkpoint for ILC1-mediated antitumor immunity
Type 1 innate lymphoid cells (ILC1s) are a class of tissue-resident cells with antitumor activity, suggesting its possible role in solid tumor immune surveillance, but it is not clear whether manipulating ILC1s can induce potent antitumor immune responses. Here, we found that G-protein-coupled receptor 34 (GPR34), a receptor for lysophosphatidylserine (LysoPS), was highly expressed on ILC1s but not on conventional natural killer cells in the tumor microenvironment. LysoPS was enriched in the tumor microenvironment and could inhibit ILC1 activation via GPR34. Genetic deletion of LysoPS synthase Abhd16a expression in tumors or Gpr34 expression in ILC1s or antagonizing GPR34 enhanced ILC1 antitumor activity. In individuals with cancer, ABHD16A expression in tumors or GPR34 expression in ILC1s was inversely correlated with the antitumor activity of ILC1s or ILC1-like cells. Thus, our results demonstrate that manipulating ILC1s can induce potent antitumor immunity, and GPR34 is a metabolic immune checkpoint that can be targeted to develop ILC1-based immunotherapy. Zhou and colleagues find that GPR34 is a metabolic immune checkpoint for ILC1-mediated antitumor immunity.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Immunology
医学-免疫学
CiteScore
40.00
自引率
2.30%
发文量
248
审稿时长
4-8 weeks
期刊介绍:
Nature Immunology is a monthly journal that publishes the highest quality research in all areas of immunology. The editorial decisions are made by a team of full-time professional editors. The journal prioritizes work that provides translational and/or fundamental insight into the workings of the immune system. It covers a wide range of topics including innate immunity and inflammation, development, immune receptors, signaling and apoptosis, antigen presentation, gene regulation and recombination, cellular and systemic immunity, vaccines, immune tolerance, autoimmunity, tumor immunology, and microbial immunopathology. In addition to publishing significant original research, Nature Immunology also includes comments, News and Views, research highlights, matters arising from readers, and reviews of the literature. The journal serves as a major conduit of top-quality information for the immunology community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


