钴催化的烯烃和对称 1,3-二酮的对映选择性氢醚化反应
IF 11.6
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
烯烃的催化不对称加氢乙醚化是一种有效的策略,可以从容易获得的起始材料中获得对映体富氧构件。然而,简单烯烃的对映体选择性分子间转化技术尤其欠发达。本文介绍了一种由 Co(III)酸酐介导的对映体选择性烯烃氢醚化反应,该反应是通过自由基-极性交叉 H 原子转移进行的,并以环状 1,3-二酮衍生物作为 O 型亲核伙伴。这种实用的方法适用于具有良好官能团耐受性的苯乙烯和脂肪烯烃,可以方便地获得结构多样的手性乙烯基酯衍生物,并具有极佳的区域、化学和对映选择性。理论研究表明,烷基 Co(III) 中间体的形成和烷基 Co(IV) 与亲核物的 SN2 取代会影响产物的立体选择性。此外,与 C-C 键构建过程中不利的立体阻碍相比,底物分子的 -OH 分子与沙林配体之间的 O-H---π 相互作用在决定独特的不对称 C-O 键化学选择性方面起着至关重要的作用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
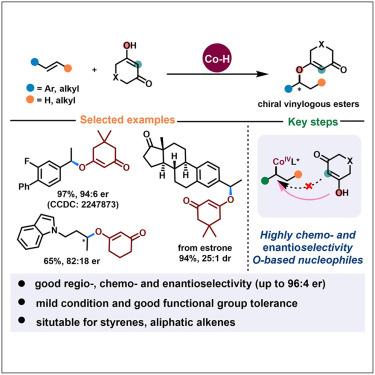
Cobalt-catalyzed enantioselective hydroetherification of alkenes and symmetric 1,3-diketones
Catalytic asymmetric hydroetherification of alkenes constitutes an efficient strategy toward enantioenriched oxygenated building blocks from readily available starting materials. However, the enantioselective intermolecular transformation of simple alkenes is particularly underdeveloped. Here, a Co(III)-hydride-mediated enantioselective olefin hydroetherification through radical-polar crossover H atom transfer has been described, with cyclic 1,3-diketone derivatives as O nucleophilic partners. This practical method is applicable for both styrenes and aliphatic alkenes with good functional group tolerance, enabling facile access to structurally diverse chiral vinylogous ester derivatives with excellent regio-, chemo-, and enantioselectivity. Theoretical studies have shown that the formation of alkyl Co(III) intermediates and the SN2-substitution of alkyl Co(IV) with nucleophiles have an effect on the stereoselectivity of the products. Additionally, the O–H···π interaction between the –OH moiety of substrate moiety and salen ligand plays a crucial role in determining unique asymmetric C–O bond chemoselectivity compared to the disfavored steric hindrance in C–C bond construction.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
10.50
自引率
6.40%
发文量
0
期刊介绍:
Chem Catalysis is a monthly journal that publishes innovative research on fundamental and applied catalysis, providing a platform for researchers across chemistry, chemical engineering, and related fields. It serves as a premier resource for scientists and engineers in academia and industry, covering heterogeneous, homogeneous, and biocatalysis. Emphasizing transformative methods and technologies, the journal aims to advance understanding, introduce novel catalysts, and connect fundamental insights to real-world applications for societal benefit.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


