基于 NiFe 双金属位点的不对称桥式结构,用于优化硝酸盐还原反应
IF 11.5
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
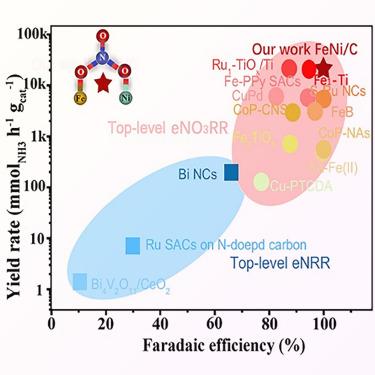
在分子水平上实现对催化剂反应过程的精确控制和准确理解,对于挖掘硝酸盐还原反应(NO3RR)的理想材料来说仍然具有挑战性和关键性。考虑到非贵金属催化剂的代表性及其 d 电子态的可调节性,研究人员合成了不同含量比的 NiFe 双金属纳米催化剂,以评估它们在电化学 NO3RR 反应中的积极特性。最终,倾向于立方 FeNi3 结构的 Ni0.7Fe0.3 样品被证明具有最佳的 NO3RR 电催化性能:在 1 M KNO3 条件下,其产氨效率(APE)为 13.61 mol g-1 h-1 ,法拉第效率(FE)为 99.53%;在 1 mM KNO3 条件下,其产氨效率(APE)为 47.94 mmol g-1 h-1 ,法拉第效率(FE)为 95.04%,优于大多数已报道的电催化剂。此外,上述数据还概述了受 NiFe 基不对称桥结构限制的独特含氮中间体的完全演化过程。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Asymmetric bridge structure based on NiFe bimetallic sites for optimizing nitrate reduction reaction
Achieving precise control and an accurate understanding of reaction processes that occur on catalysts at the molecular level is still challenging yet crucial to digging out ideal materials for the nitrate reduction reaction (NO3RR). Considering the representativeness of non-precious metal catalysts and the adjustability of their d electronic states, NiFe bimetallic nanocatalysts with various content ratios were synthesized to evaluate their positive characteristics on the electrochemical NO3RR. Eventually, the Ni0.7Fe0.3 sample inclined toward the cubic FeNi3 structure was proven to have the best electrocatalytic NO3RR performance: 13.61 mol g−1 h−1 ammonia production efficiency (APE) and 99.53% Faradaic efficiency (FE) under 1 M KNO3 and 47.94 mmol g−1 h−1 APE and 95.04% FE under 1 mM KNO3, superior to most reported electrocatalysts. Moreover, the above data outline the complete evolution of the distinct nitrogen-containing intermediate restricted by the NiFe-based asymmetric bridge structure.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
10.50
自引率
6.40%
发文量
0
期刊介绍:
Chem Catalysis is a monthly journal that publishes innovative research on fundamental and applied catalysis, providing a platform for researchers across chemistry, chemical engineering, and related fields. It serves as a premier resource for scientists and engineers in academia and industry, covering heterogeneous, homogeneous, and biocatalysis. Emphasizing transformative methods and technologies, the journal aims to advance understanding, introduce novel catalysts, and connect fundamental insights to real-world applications for societal benefit.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


