设计基于 LiTFSI 的双盐电解质的溶剂-阴离子相互作用,以维持高性能 NCM622 阴极
IF 18.9
1区 材料科学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
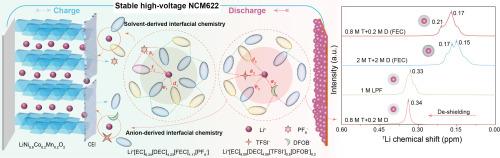
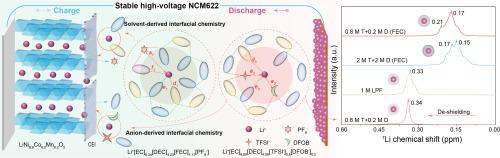
电解质在决定锂离子电池(LIB)性能方面起着至关重要的作用,尤其是在基于 LiTFSI 的电解质中的高压 LiNi0.6Co0.2Mn0.2O2 (NCM622) 正极。在此,我们报告了一种高容量、稳定的 NCM622 阴极,它可以在基于 LiTFSI(命名为 T)的碳酸盐电解质中实现,方法是使用添加的 LiDFOB(命名为 D)调整分子间的相互作用。在 1 M 双盐电解质中,4.5 V-NCM622||Li 电池的阴极失效和铝腐蚀得到了抑制,这是因为建立了均匀的界面层和较弱的 Li+-溶剂相互作用,从而抑制了寄生反应。因此,在 1 M T+D 双盐电解液中,200 次循环后的容量保持率达到 94.68%,10 C 速率容量约为 160 mA h g-1。与常用电解液不同的是,FEC 添加剂增加了(T+D)双盐体系中的脱溶障碍,干扰了循环稳定性。密度泛函理论(DFT)计算和核磁共振(NMR)光谱显示,添加剂 FEC 改变了 T+D 存在时溶剂-溶剂和溶剂-阴离子之间的相互作用,从而削弱了电解质-阴极的相容性。这项工作表明,从溶剂-溶剂/阴离子相互作用中调节溶解结构和界面化学性质,对于利用双盐电解质设计高性能锂离子电池大有可为。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Engineering the solvent-anion interactions of LiTFSI-based dual-salt electrolytes to sustain high-performance NCM622 cathodes
Electrolytes play a vital role in determining the performances of lithium-ion batteries (LIBs), especially the high-voltage LiNi0.6Co0.2Mn0.2O2 (NCM622) cathode in LiTFSI-based electrolytes. Herein, we report a high-capacity and stable NCM622 cathode that can be realized in LiTFSI-based (named T) carbonate electrolytes by tuning the intermolecular interactions using the added LiDFOB (named D). In the 1 M dual-salt electrolyte, the cathode failure and Al corrosion are suppressed in the 4.5 V-NCM622||Li batteries, because a uniform interfacial layer and weaker Li+-solvent interactions are built to inhibit the parasitic reactions. As a result, the capacity retention reaches 94.68% after 200 cycles and the 10 C-rate capacity is about 160 mA h g-1 in the 1 M T + D dual-salt electrolyte. Unlike the commonly used electrolyte, the FEC additive increases the de-solvation barrier and disturbs the cycle stability in (T + D) dual-salt systems. Density functional theory (DFT) calculation and nuclear magnetic resonance (NMR) spectra reveal that the additive FEC changes the solvent-solvent and solvent-anion interactions in the presence of T + D, which weakens the electrolyte-cathode compatibility. This work indicates that regulating the solvation structure and interfacial chemistry from solvent-solvent/anion interactions is promising for designing high-performance LIBs by using dual‐salt electrolytes.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Energy Storage Materials
Materials Science-General Materials Science
CiteScore
33.00
自引率
5.90%
发文量
652
审稿时长
27 days
期刊介绍:
Energy Storage Materials is a global interdisciplinary journal dedicated to sharing scientific and technological advancements in materials and devices for advanced energy storage and related energy conversion, such as in metal-O2 batteries. The journal features comprehensive research articles, including full papers and short communications, as well as authoritative feature articles and reviews by leading experts in the field.
Energy Storage Materials covers a wide range of topics, including the synthesis, fabrication, structure, properties, performance, and technological applications of energy storage materials. Additionally, the journal explores strategies, policies, and developments in the field of energy storage materials and devices for sustainable energy.
Published papers are selected based on their scientific and technological significance, their ability to provide valuable new knowledge, and their relevance to the international research community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


