核线粒体呼吸蛋白的选择性翻译重塑了琥珀酸代谢在急性髓细胞性白血病发展和化疗抗药性过程中的作用
IF 19.8
1区 医学
Q1 CELL & TISSUE ENGINEERING
引用次数: 0
摘要
线粒体的适应性动态地重塑了细胞的生物能和新陈代谢,并赋予了人类癌症的关键特性。然而,这些线粒体反应的选择性调控在很大程度上仍然难以捉摸。在这里,受急性髓性白血病(AML)基因筛选的启发,我们发现 RAS 效应子 RREB1 是一种翻译调节因子,并揭示了人类癌症中核编码线粒体蛋白的独特翻译控制系统。RREB1 缺失会降低线粒体活性和琥珀酸代谢,从而损害白血病干细胞(LSC)功能和急性髓细胞性白血病的发展。补充复合体 II 亚基 SDHD 可以纠正这些缺陷。值得注意的是,抑制复合体II可使AML细胞对venetoclax治疗重新敏感。从机理上讲,短RREB1变体与3′UTR中的保守基团结合,并与延伸因子eEF1A1合作,增强核编码线粒体mRNA的蛋白质翻译。总之,我们的研究结果揭示了线粒体适应性在急性髓细胞性白血病发病机制中的独特翻译控制机制,并为靶向 LSCs 的这一脆弱性提供了潜在的策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
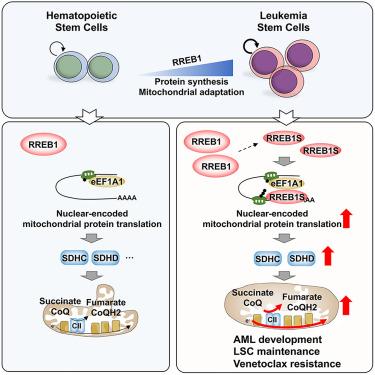
Selective translation of nuclear mitochondrial respiratory proteins reprograms succinate metabolism in AML development and chemoresistance
Mitochondrial adaptations dynamically reprogram cellular bioenergetics and metabolism and confer key properties for human cancers. However, the selective regulation of these mitochondrial responses remains largely elusive. Here, inspired by a genetic screening in acute myeloid leukemia (AML), we identify RAS effector RREB1 as a translational regulator and uncover a unique translation control system for nuclear-encoded mitochondrial proteins in human cancers. RREB1 deletion reduces mitochondrial activities and succinate metabolism, thereby damaging leukemia stem cell (LSC) function and AML development. Replenishing complex II subunit SDHD rectifies these deficiencies. Notably, inhibition of complex II re-sensitizes AML cells to venetoclax treatment. Mechanistically, a short RREB1 variant binds to a conserved motif in the 3′ UTRs and cooperates with elongation factor eEF1A1 to enhance protein translation of nuclear-encoded mitochondrial mRNAs. Overall, our findings reveal a unique translation control mechanism for mitochondrial adaptations in AML pathogenesis and provide a potential strategy for targeting this vulnerability of LSCs.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cell stem cell
生物-细胞生物学
CiteScore
37.10
自引率
2.50%
发文量
151
审稿时长
42 days
期刊介绍:
Cell Stem Cell is a comprehensive journal covering the entire spectrum of stem cell biology. It encompasses various topics, including embryonic stem cells, pluripotency, germline stem cells, tissue-specific stem cells, differentiation, epigenetics, genomics, cancer stem cells, stem cell niches, disease models, nuclear transfer technology, bioengineering, drug discovery, in vivo imaging, therapeutic applications, regenerative medicine, clinical insights, research policies, ethical considerations, and technical innovations. The journal welcomes studies from any model system providing insights into stem cell biology, with a focus on human stem cells. It publishes research reports of significant importance, along with review and analysis articles covering diverse aspects of stem cell research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


