从离子吸附稀土尾矿中高效回收稀土元素:基于添加黄铁矿的煅烧改性
IF 8.1
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
摘要
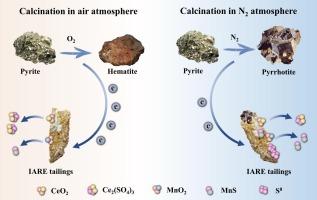
稀土尾矿是工业 (NH4)2SO4 浸出后残留的大量固体废物,通常含有稀土元素 (REE),主要是铈。本研究旨在通过在空气和二氧化氮气氛下煅烧黄铁矿,然后进行稀 H2SO4 浸出或生物浸出,从稀土尾矿中完全回收稀土元素。XRD、SEM-EDS、TIMA、SEM-BSE、XPS 和 ICP-MS 分析结果表明,铈和锰主要分别以 CeO2 和 MnO2 的形式存在,而铁则以 Fe2O3 和 Fe2(SO4)3 两种形式存在。用稀 H2SO4 进行的浸出实验表明,稀土尾矿与黄铁矿的共煅烧可显著提高稀土元素的回收率。值得注意的是,在空气中煅烧的萃取率(95.55%)明显高于在氮气中煅烧的萃取率(83.82%)。这种差异归因于氧气的存在,它促进了黄铁矿的氧化,并有利于将 Ce(IV) 还原成 Ce(III)。相反,黄铁矿在 N2 大气中发生不完全氧化,导致尾矿中的残余 Ce(IV) 含量较高,无法被稀酸浸出。随后,通过使用酸性硫杆菌铁氧体和黄铁矿进行生物浸出,从在 N2 大气中煅烧的尾矿中获得了足够的 Ce 回收率(97.52%)。因此,这项研究为从含有二氧化 Ce 的采矿尾矿或二次资源中高效回收稀土元素提供了理论支持。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Efficient recovery of rare earth elements from ion-adsorption rare earth tailings: Based on the addition of pyrite calcination modification
Rare earth tailings constitute a significant amount of solid waste that remains after industrial (NH4)2SO4 leaching and typically contain rare earth elements (REEs), primarily Ce. This study aimed to fully recover REEs from rare earth tailings by calcination with pyrite under both air and N2 atmospheres, followed by dilute H2SO4 leaching or bioleaching. The results of XRD, SEM-EDS, TIMA, SEM-BSE, XPS, and ICP-MS analyses indicated that Ce and Mn were mainly present in the form of CeO2 and MnO2, respectively, whereas Fe existed in both Fe2O3 and Fe2(SO4)3 forms. Leaching experiments with dilute H2SO4 demonstrated that the co-calcination of rare earth tailings with pyrite significantly enhanced the recovery of REEs. Notably, calcination in air resulted in a significantly higher extraction rate of Ce (95.55 %) than that in N2 (83.82 %). This difference was attributed to the presence of O2, which promoted the oxidation of pyrite and facilitated the reduction of Ce(IV) to Ce(III). In contrast, incomplete oxidation of pyrite occurred in the N2 atmosphere, leading to a high residual Ce(IV) content in the tailings that could not be leached by the dilute acid. Subsequently, sufficient recovery of Ce (97.52 %) from the tailings calcined in the N2 atmosphere was achieved by bioleaching using Acidithiobacillus ferrooxidans and pyrite. Thus, this study provides theoretical support for the efficient recovery of REEs from mining tailings or secondary sources containing CeO2.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Separation and Purification Technology
工程技术-工程:化工
CiteScore
14.00
自引率
12.80%
发文量
2347
审稿时长
43 days
期刊介绍:
Separation and Purification Technology is a premier journal committed to sharing innovative methods for separation and purification in chemical and environmental engineering, encompassing both homogeneous solutions and heterogeneous mixtures. Our scope includes the separation and/or purification of liquids, vapors, and gases, as well as carbon capture and separation techniques. However, it's important to note that methods solely intended for analytical purposes are not within the scope of the journal. Additionally, disciplines such as soil science, polymer science, and metallurgy fall outside the purview of Separation and Purification Technology. Join us in advancing the field of separation and purification methods for sustainable solutions in chemical and environmental engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


