结晶取向对锗酸锂(Li2GeO3)阳极表面性质和离子传输的作用:计算方法
IF 3.2
3区 化学
Q2 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
摘要
锂离子电池(LIB)的性能取决于其阳极的表面特性。与块体材料相比,阳极表面在锂(Li)吸收和释放过程中更容易受到环境变化的影响,从而直接影响容量、循环稳定性和充放电率等因素。锗酸锂(Li2GeO3,LGO)因其锂离子传导速度快而成为一种前景广阔的负极材料。虽然许多研究都在探索通过各种方法(包括缺陷工程)来提高性能。然而,目前还缺乏对表面结构的原子级理解。因此,尽管精确了解表面对操纵其不同性能非常重要,但 LGO 的具体表面细节仍不清楚。本研究利用理论计算来预测 LGO 表面的结构、电化学特性和锂离子传输行为,从而满足了这一关键需求。我们的研究结果表明,与非极性表面相比,极性表面的形成能更低。进一步研究发现,在各种表面端接中,锂端接表面的表面能最低。有趣的是,功函数计算显示出与表面形成能相反的趋势,极性表面的功函数值最低。值得注意的是,在所有考虑过的表面中,(003) 表面的锂离子扩散速率最高。进一步分析(001) 表面,发现其扩散途径与 (003) 表面相似,但扩散速率较低。尽管路径相似,(003) 表面的能垒明显低于(001) 表面。这一发现表明,表面的内在能谱在决定锂离子迁移行为方面起着至关重要的作用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
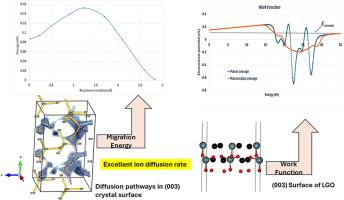
The role of crystallographic orientation on surface properties and ion transport in lithium germanate (Li2GeO3) anodes: A computational approach
The performance of lithium-ion batteries (LIBs) hinges on the surface properties of their anodes. Compared to the bulk material, the anode surface is more susceptible to environmental changes during lithium (Li) intake and release, directly impacting factors like capacity, cycling stability, and charge/discharge rates. Lithium germanate (Li2GeO3, LGO) has emerged as a promising anode material due to its fast Li-ion conduction. While numerous studies have explored performance improvements through various methods, including defect engineering. However, there is currently a lack of atomistic-level understanding of the surface structure. Consequently, despite the importance of precisely understanding the surface to manipulate its different properties, specific surface details of LGO remain unclear. This knowledge gap hinders precise manipulation of surface properties for optimal performance.This study addresses this critical need by employing theoretical calculations to predict the structural, electrochemical characteristics, and Li-ion transport behavior in LGO surfaces. Our results indicate that polar surfaces exhibit lower formation energies compared to non-polar surfaces. Further investigation revealed that Li-terminated surfaces possess the lowest surface energy among various surface terminations. Interestingly, the work function calculations displayed an opposite trend to surface formation energy, with polar surfaces exhibiting the lowest work function values.To explore Li-ion transport, we employed ab initio molecular dynamics simulations. Notably, the (003) surface displayed the highest Li-ion diffusion rate among all considered surfaces.Further analysis of the (001) surface, which exhibited similar diffusion pathways to the (003) surface, revealed a lower diffusion rate.To understand this disparity, nudged elastic band (NEB) simulations were used to estimate the energy barriers for Li-ion migration along each pathway in both structures. Despite sharing similar pathways, the energy barriers in the (003) surface were significantly lower than those in the (001) surface. This finding suggests that the intrinsic energy landscape of the surface plays a crucial role in dictating Li-ion transport behavior.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Solid State Chemistry
化学-无机化学与核化学
CiteScore
6.00
自引率
9.10%
发文量
848
审稿时长
25 days
期刊介绍:
Covering major developments in the field of solid state chemistry and related areas such as ceramics and amorphous materials, the Journal of Solid State Chemistry features studies of chemical, structural, thermodynamic, electronic, magnetic, and optical properties and processes in solids.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


