乙醇在模型 Pt(111) 单晶基底上电沉积银的初始阶段
IF 4.1
3区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
摘要
深共晶溶剂(DES)是一类具有独特性质的新型溶剂,是非常有前途的金属和合金电镀介质。通过对具有已知有序表面结构的模型单晶电极进行研究,可以明显促进对金属电沉积规律性的了解。在这项工作中,我们研究了氯化银乙醇溶液中的银在铂(111)单晶表面的欠电位沉积和过电位沉积(分别为upd和opd)的规律性。为了更好地理解发生的过程,我们采用了一种涉及电化学、原位和非原位显微技术的复杂方法。我们发现,在尚未观察到大规模溶剂降解的电位范围内,Pt(111)表面出现了逐渐中毒的过程。我们还观察到,这一过程的程度在很大程度上取决于所使用的电极与溶液之间的接触电位以及所研究的电位范围。现有结果和电荷分析用于比较乙醇和水溶液中的银更新过程。我们讨论了铂(111)上银吸附层的数量、电极与溶液初次接触的电位和循环历史的影响,以及阴离子更新和银原子与更新的氯阴离子(氯原子)之间形成共吸附剂的可能性。我们还证明,更新的银直到高阳极电位才会完全解吸,在高阳极电位下,更新的银似乎会催化溶剂氧化。在过电位几乎为零的情况下,铂(111)上的乙碱银开始相沉积,这可能与已经沉积的银吸附层的存在有关。即使在很低的过电位下,沉积物也包括在表面缺陷和平坦沉积岛边缘形成的大型平面二维结晶和三维结晶。随着过电势的增加,外延性逐渐消失;沉积物变得不规则,三维晶粒的数量和高度增加。本文章由计算机程序翻译,如有差异,请以英文原文为准。
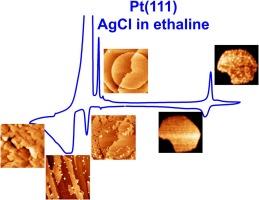
Initial stages of silver electrodeposition on a model Pt(111) single-crystal substrate from ethaline
Deep eutectic solvents (DES) are a new class of solvents with unique properties that are very promising media for the electroplating of metals and alloys. Knowledge about the regularities of metal electrodeposition is noticeably promoted by studies on model single crystal electrodes with a known well-ordered surface structure. In this work, we investigate the regularities of under- and overpotential deposition (upd and opd, respectively) of silver from a silver chloride solution in ethaline on a Pt(111) single crystal surface. For a better understanding of the occurring processes, we employ a complex approach involving electrochemical and in situ and ex situ microscopic techniques. We show that the process of gradual surface poisoning is observed on Pt(111) in the potential range in which no massive solvent degradation is yet observed. We also observe that the extent of this process largely depends on the employed potential of contact between the electrode and the solution and the studied potential range.
The presence of Ag upd on Pt(111) in ethaline is demonstrated using voltammetric and in situ STM data. The available results and charge analysis are used to compare the Ag upd processes in ethaline and in aqueous solutions. We discuss the number of Ag adlayers on Pt(111), the effect of the potential of initial contact of the electrode with the solution and cycling history, and the possibility of anion upd and formation of coadsorbate between Ag adatoms and upd chloride anions (chlorine adatoms). We also demonstrate that upd Ag is not completely desorbed until high anodic potentials, where it seems to catalyze the solvent oxidation. Phase deposition of silver from ethaline on Pt(111) starts at almost zero overpotentials, which is probably related to the presence of the already deposited Ag adlayer. Even at very low overpotentials the deposit includes both large planar 2D crystallites and 3D crystallites formed on the surface defects and at the edges of the flat deposit islands. As the overpotential grows, the epitaxiality is gradually lost; the deposit becomes unshaped and the amount of 3D crystallites and their height increase.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
7.80
自引率
6.70%
发文量
912
审稿时长
2.4 months
期刊介绍:
The Journal of Electroanalytical Chemistry is the foremost international journal devoted to the interdisciplinary subject of electrochemistry in all its aspects, theoretical as well as applied.
Electrochemistry is a wide ranging area that is in a state of continuous evolution. Rather than compiling a long list of topics covered by the Journal, the editors would like to draw particular attention to the key issues of novelty, topicality and quality. Papers should present new and interesting electrochemical science in a way that is accessible to the reader. The presentation and discussion should be at a level that is consistent with the international status of the Journal. Reports describing the application of well-established techniques to problems that are essentially technical will not be accepted. Similarly, papers that report observations but fail to provide adequate interpretation will be rejected by the Editors. Papers dealing with technical electrochemistry should be submitted to other specialist journals unless the authors can show that their work provides substantially new insights into electrochemical processes.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


