免疫介导的炎症性疾病背景下脂肪肝的临床和分子特征描述
IF 7.5
1区 医学
Q1 GASTROENTEROLOGY & HEPATOLOGY
引用次数: 0
摘要
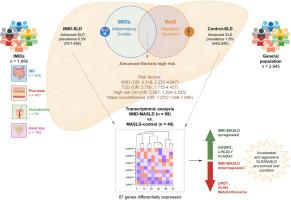
背景& 目的越来越多的证据表明,在免疫介导的炎症性疾病(IMID)的背景下,代谢功能障碍相关性脂肪性肝病(MASLD)的发病率越来越高。我们的目的是从临床和机理上对前瞻性队列中的 IMID 患者与对照组进行脂肪肝(SLD)比较。来自普通人群的对照组在年龄、性别、2 型糖尿病和体重指数方面均按 1:2 的比例进行了匹配。使用受控衰减参数确定 SLD。如果怀疑有明显的肝纤维化,则进行肝活检。从新鲜冷冻病例中提取总 RNA,并进行 RNA-seq 分析。使用 "limma-voom "进行差异基因表达分析。使用fgsea R软件包和预先排序的 "limma t-statistic "基因列表进行了基因集富集分析。IMID患者的晚期SLD(肝脏硬度测量值≥9.7 kPa)(13.46% vs. 3.79%; p <0.001)和晚期MASLD(12.8% vs. 2.8%; p <0.001)患病率明显高于对照组。在多变量分析中,并发 IMID 是晚期 SLD 的独立且最强的预测因素(调整后的几率比 3.318; 95% CI 2.225-4.947; p <0.001)。研究人员获得了109例患者的转录组数据,结果显示IMID-MASLD与对照组-MASLD之间有87个显著的基因表达差异。IMID-MASLD病例中与促肿瘤活动或细胞周期控制有关的基因表达丰富,而与新陈代谢有关的基因表达为负。我们的研究结果表明,免疫介导的炎症性疾病可能会催化一种不同于传统代谢途径的独特的MASLD途径,这就凸显了制定有针对性的临床管理策略的必要性。影响和意义免疫介导的炎症性疾病患者中伴有晚期纤维化的脂肪性肝病发病率增加,与传统的代谢风险因素或高风险饮酒无关。转录组分析揭示了一种独特的基因表达特征,这种特征与导致脂肪肝的加速和侵袭性形式的肝病的细胞活动有关。我们的研究结果表明,对免疫介导的炎症性疾病患者的不同医学领域加强晚期肝病风险筛查非常重要。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Clinical and molecular characterization of steatotic liver disease in the setting of immune-mediated inflammatory diseases
Background & Aims
Growing evidence suggests an increased prevalence of metabolic dysfunction-associated steatotic liver disease (MASLD) in the context of immune-mediated inflammatory diseases (IMIDs). We aimed to clinically and mechanistically characterize steatotic liver disease (SLD) in a prospective cohort of patients with IMID compared to controls.
Methods
Cross-sectional, case-control study including a subset of patients with IMID. Controls from the general population were age-, sex-, type 2 diabetes-, and BMI-matched at a 1:2 ratio. SLD was established using controlled attenuation parameter. Liver biopsies were obtained when significant liver fibrosis was suspected. Total RNA was extracted from freshly frozen cases and analyzed by RNA-seq. Differential gene expression was performed with ‘limma-voom’. Gene set-enrichment analysis was performed using the fgsea R package with a preranked “limma t-statistic” gene list.
Results
A total of 1,456 patients with IMID and 2,945 controls were included. Advanced SLD (liver stiffness measurement ≥9.7 kPa) (13.46% vs. 3.79%; p <0.001) and advanced MASLD (12.8% vs. 2.8%; p <0.001) prevalence were significantly higher among patients with IMID than controls. In multivariate analysis, concomitant IMID was an independent, and the strongest, predictor of advanced SLD (adjusted odds ratio 3.318; 95% CI 2.225-4.947; p <0.001). Transcriptomic data was obtained in 109 patients and showed 87 significant genes differentially expressed between IMID-MASLD and control-MASLD. IMID-MASLD cases displayed an enriched expression of genes implicated in pro-tumoral activities or the control of the cell cycle concomitant with a negative expression of genes related to metabolism.
Conclusions
The prevalence of advanced SLD and MASLD is disproportionately elevated in IMID cohorts. Our findings suggest that IMIDs may catalyze a distinct MASLD pathway, divergent from classical metabolic routes, highlighting the need for tailored clinical management strategies.
Impact and implications
The prevalence of steatotic liver disease with advanced fibrosis is increased in patients with immune-mediated inflammatory diseases, independent of classic metabolic risk factors or high-risk alcohol consumption. Transcriptomic analysis revealed a unique gene expression signature associated with cellular activities that are compatible with a liver condition leading to an accelerated and aggressive form of steatotic liver disease. Our findings underscore the importance of heightened screening for advanced liver disease risk across various medical disciplines overseeing patients with immune-mediated inflammatory diseases.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

JHEP Reports
GASTROENTEROLOGY & HEPATOLOGY-
CiteScore
12.40
自引率
2.40%
发文量
161
审稿时长
36 days
期刊介绍:
JHEP Reports is an open access journal that is affiliated with the European Association for the Study of the Liver (EASL). It serves as a companion journal to the highly respected Journal of Hepatology.
The primary objective of JHEP Reports is to publish original papers and reviews that contribute to the advancement of knowledge in the field of liver diseases. The journal covers a wide range of topics, including basic, translational, and clinical research. It also focuses on global issues in hepatology, with particular emphasis on areas such as clinical trials, novel diagnostics, precision medicine and therapeutics, cancer research, cellular and molecular studies, artificial intelligence, microbiome research, epidemiology, and cutting-edge technologies.
In summary, JHEP Reports is dedicated to promoting scientific discoveries and innovations in liver diseases through the publication of high-quality research papers and reviews covering various aspects of hepatology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


