生物质粉煤灰和沼气残渣热解炭对 Cu (II) 的吸附行为和机理的系统比较
Q1 Environmental Science
引用次数: 0
摘要
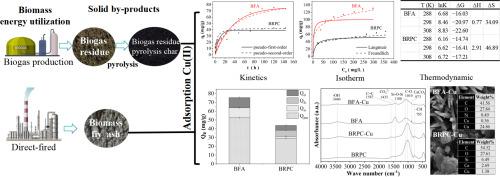
生物质粉煤灰(BFA)和沼气渣是生物质直燃发电和沼气生产的主要副产品。本研究通过系统测试比较了生物质粉煤灰和沼气残渣热解炭(BRPC)对 Cu(II)的吸附机理。BFA 的吸附容量(75.34 mg/g)明显高于 BRPC(42.80 mg/g)。伪一阶动力学模型最适合描述 BFA,而 Elovich 模型最适合描述 BRPC。两种材料都符合 Freundlich 等温线模型。BFA 具有优异的矿物共沉淀和离子交换能力,这是因为它含有丰富的矿物质,尤其是钙。由于含有热解木炭,BRPC 还具有额外的官能团复合吸附能力。BFA 和 BRPC 的孔隙结构变化对吸附作用没有明显影响。这项研究的结果为 BFA 和 BRPC 的高值化利用潜力以及高性能生物质重金属吸附材料的创新改性提供了重要的数据支持。本文章由计算机程序翻译,如有差异,请以英文原文为准。
A systematical comparation of Cu (II) adsorption behavior and mechanism between biomass fly ash and biogas residue pyrolysis char
Biomass fly ash (BFA) and biogas residues are the main by-products of biomass direct-fired power generation and biogas production. This study compares the Cu(II) adsorption mechanisms of BFA and biogas residue pyrolysis char (BRPC) through systematic tests. BFA exhibited a significantly higher adsorption capacity (75.34 mg/g) than BRPC (42.80 mg/g). The pseudo-first-order kinetic model best described BFA, while the Elovich model was optimal for BRPC. Both materials fit the Freundlich isotherm model. BFA's superior mineral co-precipitation and ion-exchange capacity are due to its rich mineral content, particularly calcium. BRPC benefits from an additional functional group complex adsorption due to its pyrolytic charcoal content. Variations in pore structure of BFA and BRPC did not demonstrate a significant effect on the adsorption. The results of this study provide essential data support for the potential of high-value utilization of BFA and BRPC and the innovative modification of high-performance biomass-based heavy metal adsorbent materials.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Bioresource Technology Reports
Environmental Science-Environmental Engineering
CiteScore
7.20
自引率
0.00%
发文量
390
审稿时长
28 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


