氧化铜修饰的黄铁矿煤渣氧载体在化学循环燃烧中的性能提升
IF 8.1
2区 工程技术
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
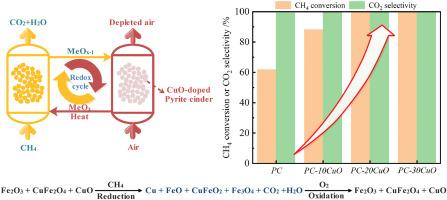
由于活性成分丰富且成本低廉,黄铁矿煤渣在化学循环燃烧(CLC)过程中作为氧载体具有巨大潜力。为了提高燃料的燃烧反应性,我们用氧化铜(CuO)对黄铁矿煤渣进行了改性。氧化铜的加入可有效增加黄铁矿煤渣的孔隙体积和氧空位浓度。含 20 wt% CuO 的改性黄铁矿煤渣具有最高的孔隙体积(0.3 cm3/g)和氧空隙浓度(55.61%)。在长期氧化还原循环过程中,改性黄铁矿煤渣样品比未掺杂样品显示出更高的燃料燃烧反应性。表征结果表明,在 CuO 改性黄铁矿煤渣样品中形成了 CuO 和 CuFe2O4 结晶相。改性黄铁矿煤渣样品中的活性成分(Fe2O3、CuFe2O4 和 CuO)在还原半周期中被还原成 Fe3O4、FeO、CuFeO2 和 Cu。在多重氧化还原测试中,含 20 wt% CuO 的改性黄铁矿煤渣具有最高的 CH4 转化率(接近 100%)和 CO2 选择性(接近 100%)。而未掺杂的黄铁矿煤渣的 CH4 转化率仅为 60%左右。当 CuO 含量达到 30 wt% 时,改性黄铁矿煤渣的表面烧结,导致 CH4 转化率和表面积下降。经过连续的氧化还原循环后,CuO 改性黄铁矿煤渣样品仍能保持原有的晶体结构。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Enhanced performance of pyrite cinder oxygen carrier modified by CuO for chemical looping combustion
Due to the abundance of active components and low cost, pyrite cinder has great potential as an oxygen carrier in the process of chemical looping combustion (CLC). In order to improve the fuel combustion reactivity, we modified pyrite cinder with copper oxide (CuO). The addition of copper oxide could effectively increase the pore volume and oxygen vacancy concentration of pyrite cinder. The modified pyrite cinder with 20 wt% CuO possessed the highest pore volume (0.3 cm3/g) and oxygen vacancy concentration (55.61%). During the long-term redox cycles, the modified pyrite cinder samples showed higher fuel combustion reactivity than the undoped sample. The results of characterization indicated that the crystalline phases of CuO and CuFe2O4 were formed in the CuO-modified pyrite cinder samples. The active components (Fe2O3, CuFe2O4 and CuO) in the modified pyrite cinder samples were reduced to Fe3O4, FeO, CuFeO2 and Cu during the reduction half cycle. The modified pyrite cinder with 20 wt% CuO possessed the highest CH4 conversion (nearly 100%) and CO2 selectivity (nearly 100%) in multiple redox testing. The CH4 conversion of undoped pyrite cinder was only about 60%. When the content of CuO reached 30 wt%, surface sintering occurred for the modified pyrite cinder, resulting in the decrease of CH4 conversion and surface area. After continuous redox cycles, the CuO-modified pyrite cinder samples could maintain the original crystal structure.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

International Journal of Hydrogen Energy
工程技术-环境科学
CiteScore
13.50
自引率
25.00%
发文量
3502
审稿时长
60 days
期刊介绍:
The objective of the International Journal of Hydrogen Energy is to facilitate the exchange of new ideas, technological advancements, and research findings in the field of Hydrogen Energy among scientists and engineers worldwide. This journal showcases original research, both analytical and experimental, covering various aspects of Hydrogen Energy. These include production, storage, transmission, utilization, enabling technologies, environmental impact, economic considerations, and global perspectives on hydrogen and its carriers such as NH3, CH4, alcohols, etc.
The utilization aspect encompasses various methods such as thermochemical (combustion), photochemical, electrochemical (fuel cells), and nuclear conversion of hydrogen, hydrogen isotopes, and hydrogen carriers into thermal, mechanical, and electrical energies. The applications of these energies can be found in transportation (including aerospace), industrial, commercial, and residential sectors.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


