二氧化铈催化微通道反应器中氮氧化物辅助异相催化燃烧烟尘的 N2O 形成机理研究
IF 5.6
2区 工程技术
Q2 ENERGY & FUELS
引用次数: 0
摘要
基于 CeO2 的催化微通道反应器固定床实验研究了在 NOx 辅助异相催化燃烧烟尘过程中,新鲜催化剂和水热老化催化剂在 NOx 辅助催化燃烧过程中 N2O 的形成。基于原位傅立叶变换红外光谱(FTIR)诊断和命运函数理论(DFT)计算,建立了进化的氮氧化物辅助烟尘催化燃烧反应机理,以研究 N2O 的形成和关键反应途径。研究发现,N2O 的形成温度范围与烟尘催化燃烧的起始温度相同,而 CeO2 催化剂的显著催化活性导致 N2O 的形成温度范围减小。CeO2 催化剂可抑制 NOx 辅助烟尘催化燃烧中 N2O 的生成,但其抑制作用随催化剂活性的降低而逐渐减弱。CeO2 对 N2O 的抑制作用体现在高温下 CN 生成率的降低上。新的 CeO2 催化剂增加了 CN 生成反应的主导性,降低了 CN 的生成速率,并有助于降低 CNO 被 NO 和 NO2 氧化的反应速率。与α(NO2 与 NOx 的比率)和γ(O2 与 NOx 的比率)相比,NOx 与烟尘比率(β)的增加对 N2O 的形成更为敏感,从而导致对 N2O 形成的抑制作用更强。本文章由计算机程序翻译,如有差异,请以英文原文为准。
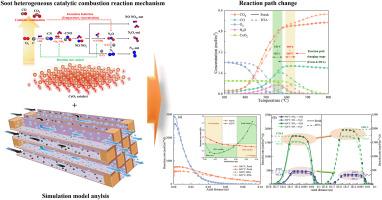
Study of the N2O formation mechanism in NOx-assisted heterogeneous catalytic combustion of soot in CeO2-based catalytic microchannel reactor
A CeO2-based catalytic microchannel reactor fixed-bed experiment was carried out to investigate the N2O formation in NOx-assisted catalytic combustion with fresh and hydrothermally aging catalysts during NOx-assisted heterogeneous catalytic combustion of soot. An evolved NOx-assisted soot catalytic combustion reaction mechanism was built to investigate N2O formation and key reaction pathways based on in situ Fourier Transform Infrared Spectroscopy (FTIR) diagnostics and destiny functional theory (DFT) computations. It was found that the temperature range of N2O formation was the same as the initiation temperature of soot catalytic combustion, while the significant catalytic activity of CeO2 catalyst induced a decrease in the temperature range of N2O formation. The CeO2 catalyst inhibited N2O formations from NOx-assisted soot catalytic combustion, while its inhibition effect was gradually weakened with the decrease of catalyst activities. The inhibitory effect of CeO2 on N2O was revealed in the reduction of CN formation rate in high temperatures. Fresh CeO2 catalyst increased the dominance in the CN formation reaction, reduced the CN production rate, and contributed to the decrease in the reaction rate of CNO oxidation by NO and NO2. The increase in the ratio of NOx to soot (β) was more sensitive to N2O formation than the ratio α (NO2 to NOx) and γ (O2 to NOx), led to a stronger inhibition of N2O formation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of The Energy Institute
工程技术-能源与燃料
CiteScore
10.60
自引率
5.30%
发文量
166
审稿时长
16 days
期刊介绍:
The Journal of the Energy Institute provides peer reviewed coverage of original high quality research on energy, engineering and technology.The coverage is broad and the main areas of interest include:
Combustion engineering and associated technologies; process heating; power generation; engines and propulsion; emissions and environmental pollution control; clean coal technologies; carbon abatement technologies
Emissions and environmental pollution control; safety and hazards;
Clean coal technologies; carbon abatement technologies, including carbon capture and storage, CCS;
Petroleum engineering and fuel quality, including storage and transport
Alternative energy sources; biomass utilisation and biomass conversion technologies; energy from waste, incineration and recycling
Energy conversion, energy recovery and energy efficiency; space heating, fuel cells, heat pumps and cooling systems
Energy storage
The journal''s coverage reflects changes in energy technology that result from the transition to more efficient energy production and end use together with reduced carbon emission.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


