Artepillin C 和 Aromadendrin 作为胶原酶和弹性蛋白酶抑制剂的体外和硅学治疗特性,以及抗卵巢癌效果和抗氧化潜力的研究
IF 3.2
4区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
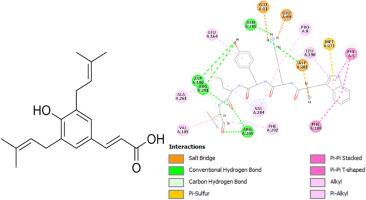
在这项研究中,青蒿素 C 和芳香树酯分子对胶原酶和弹性蛋白酶具有抑制作用,青蒿素 C 的 IC50 值分别为 16.65 和 0.79 μM,芳香树酯的 IC50 值分别为 7.31、19.38 和 10.56 μM,从优到良。通过分子模型研究,评估了这些化合物对胶原酶和弹性蛋白酶的化学活性。使用以下细胞系对这些化合物的抗癌特性进行了评估:NIH:OVCAR-3、ES-2、UACC-1598、Hs 832(C).T [Hs832.Tc]、TOV-21G、UWB1.289。本研究采用 DPPH(1,1-二苯基-2-丙基肼)自由基清除测定法和分光光度法来测定抗氧化活性。通过分子对接研究,考察了青蒿素 C 和芳香腺苷对胶原酶和弹性蛋白酶的化学活性。评估了这些化合物对 NIH:OVCAR-3、ES-2、UACC-1598、Hs 832(C).T [Hs832.Tc]、TOV-21G、UWB1.289 细胞系的抗癌活性进行了评估。此外,一些细胞株对芳香树酯的效果最好,如 NIH:OVCAR-3、ES-2、UACC-1598、Hs 832(C).T [Hs832.Tc]、TOV-21G、UWB1.289(10.54 ± 0.94、22.11 ± 2.52、31.85 ± 4.73、8.14 ± 1.52、17.94 ± 1.88 和 24.31 ± 2.64 μM)。利用分子对接计算评估了青蒿素 C 和芳香腺苷对上述细胞系中一些表达的表面受体蛋白(叶酸受体、CD44、表皮生长因子受体、甲酰基肽受体样 1、M2 肌肽受体和雌激素受体)的化学活性。计算结果提供了潜在相互作用及其原子级特性的信息。根据对接得分,这些化合物显示出与蛋白质和酶结合的高亲和力。此外,这些物质与受体和酶有良好的接触。因此,这些物质可能具有抑制癌细胞和酶的能力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
In vitro and in silico therapeutic properties of Artepillin C and Aromadendrin as collagenase and elastase inhibitors and investigation of anti-Ovarian cancer effects and antioxidant potential
In this investigation, the enzymes collagenase and elastase were inhibited by artepillin.C and aromadendrin molecules with excellent to good IC50 values of 16.65, and 0.79 μM for artepillin.C and 7.31, 19.38, and 10.56 μM for aromadendrin. Using the molecular modeling study, the chemical activity of these compounds against the enzymes, collagenase, and elastase were evaluated. The anti-cancer properties of the compounds were evaluated using the following cell lines: NIH: OVCAR-3, ES-2, UACC-1598, Hs 832(C).T [Hs832.Tc], TOV-21G, UWB1.289. The DPPH (1,1-diphenyl-2-pricrylhydrazyl) free radical scavenging assay was used to measure antioxidant activity, and the spectrophotometric method was used in this work. The chemical activities of artepillin.C and aromadendrin against collagenase and elastase were investigated utilizing the molecular docking study. The anti-cancer activities of the compounds were evaluated against NIH: OVCAR-3, ES-2, UACC-1598, Hs 832(C).T [Hs832.Tc], TOV-21G, UWB1.289 cell lines. Also, some cell lines had best results for aromadendrin like NIH: OVCAR-3, ES-2, UACC-1598, Hs 832(C).T [Hs832.Tc], TOV-21G, UWB1.289 (10.54 ± 0.94, 22.11 ± 2.52, 31.85 ± 4.73, 8.14 ± 1.52, 17.94 ± 1.88, and 24.31 ± 2.64 μM). The chemical activities of artepillin.C and aromadendrin against some of the expressed surface receptor proteins (folate receptor, CD44, EGFR, Formyl Peptide Receptor–Like 1, M2 muscarinic receptor, and estrogen receptors) in the mentioned cell lines were assessed using the molecular docking calculations. The outcomes provided information on potential interactions and their atomic-level properties. According to the docking scores, the compounds show a high affinity for binding to proteins and enzymes. Furthermore, these substances made good contact with the receptors and enzymes. As a result, these substances may have the ability to suppress cancer cells and enzymes.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
3.50
自引率
7.70%
发文量
492
审稿时长
3-8 weeks
期刊介绍:
The Journal of the Indian Chemical Society publishes original, fundamental, theorical, experimental research work of highest quality in all areas of chemistry, biochemistry, medicinal chemistry, electrochemistry, agrochemistry, chemical engineering and technology, food chemistry, environmental chemistry, etc.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


