enos的线粒体再分布受akt1和二聚体状态的调控。
IF 3.2
2区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
此前,我们已经证明,内皮一氧化氮合酶(eNOS)二聚体的水平与 eNOS 与 hsp90(热休克蛋白 90)的相互作用直接相关。此外,eNOS 二聚体的破坏与它重新分布到线粒体有关。然而,这些事件之间的因果关系还有待研究,这也是本研究的重点。我们的数据表明,辛伐他汀能减少 eNOS 的线粒体再分布,增加 eNOS-hsp90 的相互作用,并增强肺动脉高压(PH)羔羊模型培养的肺动脉内皮细胞(PAEC)中 eNOS 的二聚化。我们的数据还显示,在 hsp90 和 ATP 的存在下,人重组 eNOS 单体部分的二聚化受到了刺激。过量表达 hsp90 的显性负突变体(DNHsp90)会降低 eNOS 的二聚体水平并增强其线粒体再分布。我们还发现,过亚硝酸盐供体 3-吗啉基二亚胺(SIN-1)增加了 PAEC 中 eNOS 的线粒体再分布,这同样与 eNOS 二聚体水平的降低有关。我们的数据还显示,在 COS-7 细胞中,SIN-1 介导的野生型 eNOS(WT-eNOS)线粒体再分布明显高于二聚体稳定的 eNOS 突变蛋白(C94R/C99R-eNOS)。相反,单体 eNOS 突变蛋白(C96A-eNOS)的线粒体再分布则会增强。最后,我们将 SIN-1 介导的 eNOS 线粒体再分布与 Akt1 介导的 eNOS 丝氨酸(S)617 磷酸化联系起来,并表明该残基的磷酸化可及性受二聚化状态的调节。因此,我们的数据揭示了一种新的肺内皮功能障碍机制,它是由线粒体重新分布的 eNOS 介导的,并受二聚化状态和 S617 磷酸化的调节。本文章由计算机程序翻译,如有差异,请以英文原文为准。
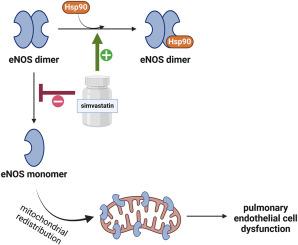
The mitochondrial redistribution of ENOS is regulated by AKT1 and dimer status
Previously, we have shown that endothelial nitric-oxide synthase (eNOS) dimer levels directly correlate with the interaction of eNOS with hsp90 (heat shock protein 90). Further, the disruption of eNOS dimerization correlates with its redistribution to the mitochondria. However, the causal link between these events has yet to be investigated and was the focus of this study. Our data demonstrates that simvastatin, which decreases the mitochondrial redistribution of eNOS, increased eNOS-hsp90 interactions and enhanced eNOS dimerization in cultured pulmonary arterial endothelial cells (PAEC) from a lamb model of pulmonary hypertension (PH). Our data also show that the dimerization of a monomeric fraction of human recombinant eNOS was stimulated in the presence of hsp90 and ATP. The over-expression of a dominant negative mutant of hsp90 (DNHsp90) decreased eNOS dimer levels and enhanced its mitochondrial redistribution. We also found that the peroxynitrite donor3-morpholinosydnonimine (SIN-1) increased the mitochondrial redistribution of eNOS in PAEC and this was again associated with decreased eNOS dimer levels. Our data also show in COS-7 cells, the SIN-1 mediated mitochondrial redistribution of wildtype eNOS (WT-eNOS) is significantly higher than a dimer stable eNOS mutant protein (C94R/C99R-eNOS). Conversely, the mitochondrial redistribution of a monomeric eNOS mutant protein (C96A-eNOS) was enhanced. Finally, we linked the SIN-1-mediated mitochondrial redistribution of eNOS to the Akt1-mediated phosphorylation of eNOS at Serine(S)617 and showed that the accessibility of this residue to phosphorylation is regulated by dimerization status. Thus, our data reveal a novel mechanism of pulmonary endothelial dysfunction mediated by mitochondrial redistribution of eNOS, regulated by dimerization status and the phosphorylation of S617.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nitric oxide : biology and chemistry
生物-生化与分子生物学
CiteScore
7.50
自引率
7.70%
发文量
74
审稿时长
52 days
期刊介绍:
Nitric Oxide includes original research, methodology papers and reviews relating to nitric oxide and other gasotransmitters such as hydrogen sulfide and carbon monoxide. Special emphasis is placed on the biological chemistry, physiology, pharmacology, enzymology and pathological significance of these molecules in human health and disease. The journal also accepts manuscripts relating to plant and microbial studies involving these molecules.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


