在环境和空间差异很大的情况下,共同感染与牡蛎的感染率和感染强度有关。
IF 3.6
3区 生物学
Q1 ZOOLOGY
引用次数: 0
摘要
寄生虫共感染会改变野生环境中的感染结果。然而,目前还不清楚多种环境因素如何影响共同感染。切萨皮克湾的东部牡蛎(Crassostrea virginica)元种群提供了一个机会,可以测试在不同环境中共同感染的重要性,因为多种寄生虫会在广泛的盐度梯度中感染牡蛎。本研究利用马里兰州和弗吉尼亚州的牡蛎监测,对四种共感染生物(包括两种组织寄生虫和两种贝壳生物侵蚀寄生虫)进行了大规模调查。我们对 16 个已收获和未收获配对礁石上的 440 只牡蛎进行了感染诊断,并测试了每种寄生虫的共感染生物与环境条件、宿主特征和海洋空间管理相关的重要性。采用显微视觉方法诊断海鲈病(dermo 病的病原体)和奈尔索尼合孢虫(MSX 病的病原体)组织感染的流行率和强度。采用宏观目测方法诊断贝壳感染 Cliona boring sponges 和水泡诱导 Polydora 蠕虫的流行程度和强度。对于检测到的三种牡蛎寄生虫(所有牡蛎均未感染 H. nelsoni),盐度是总体上最强的预测因子,与整个海湾的寄生虫流行率和/或强度模式相对应。尽管环境和空间差异很大,但共同感染与所有三种牡蛎寄生虫的流行率和/或强度的改变相对应。相关模式表明,牡蛎寄生虫在共同感染中起着关键作用,因为其强度随 Cliona 海绵的流行而增加,而牡蛎寄生虫的共同感染预示着 Polydora 水泡强度的增加。牡蛎壳高度、礁石栖息地和采收状况也预测了寄生虫的流行率和强度,进一步反映了该系统中感染的多元驱动因素。未收获的珊瑚礁具有更高的垂直生境结构和更高的克利奥纳海绵感染强度,但三种寄生虫的感染率没有差异。空间模式出乎意料地表明,珊瑚礁层面的寄生虫模式预测因素比支流之间的差异更为重要。这项相关调查通过三种牡蛎寄生虫、环境条件、宿主特征和人力资源管理之间的统计关系提供了新的见解。我们需要新的、更详细的方案来扩展疾病生态理论,以涵盖受人类影响的野生动物种群中的共同感染。本文章由计算机程序翻译,如有差异,请以英文原文为准。
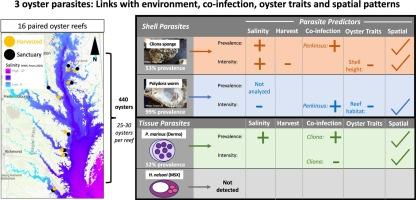
Co-infection is linked to infection prevalence and intensity in oysters amidst high environmental and spatial variation
Co-infecting parasites modify infection outcomes in the wild. However, it is unclear how multiple environmental factors influence co-infection. The Chesapeake Bay metapopulation of the eastern oyster, Crassostrea virginica, provides an opportunity to test the importance of co-infection across heterogeneous environments because multiple parasites infect oysters across a broad salinity gradient. This study leverages Maryland and Virginia oyster monitoring for a large-scale survey of four co-infecting organisms, including two tissue parasites and two shell bio-eroding parasites. We diagnosed infection in 440 oysters across 16 paired harvested and unharvested reefs and tested the importance of co-infecting organisms for each parasite relative to environmental conditions, host traits, and marine spatial management. Microscopic visual methods were used to diagnose prevalence and intensity of tissue infections with Perkinsus marinus (the causative agent of dermo disease) and Haplosporidium nelsoni (the causative agent of MSX disease). Macroscopic visual methods were used to diagnose prevalence and intensity of shell infections with Cliona boring sponges and blister-inducing Polydora worms. For the three oyster parasites that were detected [H. nelsoni infections were absent in all oysters], salinity was the overall strongest predictor, corresponding to bay-wide patterns of parasite prevalence and/or intensity. Despite high environmental and spatial variation, co-infections corresponded to altered prevalence and/or intensity for all three oyster parasites. The correlational patterns suggest that P. marinus acts as a lynchpin in co-infection, as its intensity increased with Cliona sponge prevalence and P. marinus co-infection predicted higher Polydora blister intensity. Oyster shell height, reef habitat, and harvest status also predicted parasite prevalence and intensity, further reflecting the multivariate drivers of infections in this system. Unharvested reefs had greater vertical habitat structure and higher intensities of Cliona sponge infections, but no differences in the prevalence of any of the three parasites. Spatial patterns unexpectedly show that reef-level predictors of parasite patterns were more important than differences between tributaries. This correlational survey provides novel insights through the statistical relationships between the three oyster parasites, environmental conditions, host traits, and human resource management. New and more detailed scenarios are needed to expand disease ecological theory to encompass co-infection in anthropogenically impacted wildlife populations.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
6.10
自引率
5.90%
发文量
94
审稿时长
1 months
期刊介绍:
The Journal of Invertebrate Pathology presents original research articles and notes on the induction and pathogenesis of diseases of invertebrates, including the suppression of diseases in beneficial species, and the use of diseases in controlling undesirable species. In addition, the journal publishes the results of physiological, morphological, genetic, immunological and ecological studies as related to the etiologic agents of diseases of invertebrates.
The Journal of Invertebrate Pathology is the adopted journal of the Society for Invertebrate Pathology, and is available to SIP members at a special reduced price.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


