阐明酮糖 3-酰亚胺酶中 d-阿洛糖的识别机制。
IF 2.3
4区 生物学
Q3 BIOTECHNOLOGY & APPLIED MICROBIOLOGY
引用次数: 0
摘要
d-阿洛糖是一种具有多种营养功能的低热量甜味剂,可通过酮糖3-epimerase(KEase)的d-果糖异构化作用产生。来自球形节杆菌的l-核酮糖3-epimerase(AgLRE)是产生d-阿洛糖的最重要的酶之一,但其底物识别机制尚不清楚。本研究测定了 AgLRE 及其与 d-阿洛糖和 d-果糖复合物的晶体结构。底物结合后,活性位点入口周围的疏水残基向结合的底物移动。通过比较 AgLRE 和其他 KEase 的结构发现,底物结合残基并不是 AgLRE 对 d-阿洛糖和 d-果糖具有明显特异性的主要原因,活性位点口袋的疏水性才是影响底物识别的主要因素。特别是活性位点入口处的两个疏水区域是调节 AgLRE 底物识别的调节元件。这项研究为设计 AgLRE 以提高其对 d- 阿洛糖和 d-果糖的亲和力提供了有用的信息。本文章由计算机程序翻译,如有差异,请以英文原文为准。
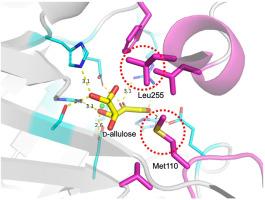
Elucidation of d-allulose recognition mechanism in ketose 3-epimerase
d-Allulose is a low-calorie sweetener with multiple nutritional functions that can be produced through d-fructose isomerization by ketose 3-epimerase (KEase). l-Ribulose 3-epimerase from Arthrobacter globiformis (AgLRE) is one of the most important enzymes that produce d-allulose; however, its substrate recognition mechanism is unknown. In this study, the crystal structures of AgLRE and its complex with d-allulose and d-fructose were determined. Upon substrate binding, the hydrophobic residues around the active-site entrance move toward the bound substrate. A comparison of AgLRE and other KEase structures revealed that the substrate-binding residues are not the main factors responsible for its marked specificity for d-allulose and d-fructose, but the hydrophobicity of the active site pocket influences substrate recognition. Particularly, the two hydrophobic regions at the active site entrance are the regulatory elements that modulate substrate recognition by AgLRE. This study provides useful information for designing AgLRE to increase its affinity for d-allulose and d-fructose.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of bioscience and bioengineering
生物-生物工程与应用微生物
CiteScore
5.90
自引率
3.60%
发文量
144
审稿时长
51 days
期刊介绍:
The Journal of Bioscience and Bioengineering is a research journal publishing original full-length research papers, reviews, and Letters to the Editor. The Journal is devoted to the advancement and dissemination of knowledge concerning fermentation technology, biochemical engineering, food technology and microbiology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


