基于 Niosome 的纳米载体的设计质量,从实验室到工业改进透皮给药。
IF 5.3
2区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
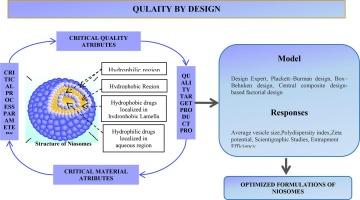
Niosomes 本质上是基于非离子表面活性剂的多胶束或单胶束囊泡。它们由排列成双分子层的表面活性剂大分子组成,双分子层包围着溶质水溶液。两亲性、可生物降解、生物相容性和环境友好型材料被用于将药物封装在囊泡中,从而提高生物利用率、疗效、药物经皮肤的渗透性,并以受控或持续的方式释放药物。以胆固醇为脂质的表面活性剂主要有吐温 20、吐温 60 和吐温 60。许多药物,包括治疗糖尿病肾病的格列本脲和抗癌药物(包括吉西他滨、顺铂和宁替达尼),都已被有效地封装到niosomes中。在实验室规模上制造 niosomes 的传统方法是薄膜水合工艺。主要成分之间的理想配比以及关键的生产工艺参数是制造具有大量药物负载和纳米形态的最佳niosomal配方的关键因素。将实验设计(DoE)和响应面方法(RSM)与质量设计(QbD)相结合,对于理解这些变量在实验室制备和放大过程中的相互作用至关重要。罗莱雅公司正在进行抗衰老化妆品的开发研究。像兰蔻这样的niosomal制剂已在商店出售。本文概述了从实验室到工业规模的niosomes、渗透机制和质量设计。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Quality by design for Niosome-Based nanocarriers to improve transdermal drug delivery from lab to industry
Niosomes are essentially multilamellar or unilamellar vesicles based on non-ionic surfactants. They consist of surfactant macromolecules arranged in a bilayer, which surrounds an aqueous solute solution. Amphiphilic, biodegradable, biocompatible, and environmentally friendly materials are utilized for encapsulating the drugs in vesicles that enhance the bioavailability, therapeutic efficacy, penetration of drug via the skin, and drug release in a controlled or sustained manner, and are employed to target the anticipated area via modifying composition that acts to minimize undesirable effects. With cholesterol as the lipid, Tween 20, Span 60, and Tween 60 are mostly employed as surfactants. Many medications, including Glibenclamide for diabetic kidney disease and anti-cancer medications including gemcitabine, cisplatin, and nintedanib, have been effectively encapsulated into niosomes. The traditional approach for creating niosomes at the lab scale is a thin film hydration process. The ideal ratio between primary components as well as critical manufacturing process parameters is key component in creating the best niosomal formulations with substantial drug loading and nanometric form. Utilizing the Design of Experiments (DoE) and Response Surface Methodology (RSM) in conjunction with Quality by design (QbD) is essential for comprehending how these variables interact both during lab preparation and during the scale-up process. Research on the development of anti-aging cosmetics is being done by Loreal. Niosomal preparations like Lancome are sold in stores. An overview of niosomes, penetration mechanisms, and quality by design from laboratory to industrial scale is provided in this article.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
10.70
自引率
8.60%
发文量
951
审稿时长
72 days
期刊介绍:
The International Journal of Pharmaceutics is the third most cited journal in the "Pharmacy & Pharmacology" category out of 366 journals, being the true home for pharmaceutical scientists concerned with the physical, chemical and biological properties of devices and delivery systems for drugs, vaccines and biologicals, including their design, manufacture and evaluation. This includes evaluation of the properties of drugs, excipients such as surfactants and polymers and novel materials. The journal has special sections on pharmaceutical nanotechnology and personalized medicines, and publishes research papers, reviews, commentaries and letters to the editor as well as special issues.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


