THz-TDS 透射测量作为片剂质量的过程分析仪。
IF 5.3
2区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
片剂的含量和含量均匀性是药品上市的关键。片剂的含量和均匀性取决于片剂中活性药物成分的重量比和片剂的总质量。目前已经提出并实施了用于控制活性药物成分比例的新型工艺分析技术工具,但仍需要更坚固、更灵敏、更快速的传感器来控制片剂质量。在本研究中,太赫兹时域光谱(THz-TDS)被提议作为一种潜在的片剂质量过程分析器。THz-TDS 以脉冲太赫兹信号为基础,并在时域中进行映射。因此,信号的振幅和到达时间都会被记录下来。使用反射装置对片剂进行 THz-TDS 测量会产生两个信号--正面反射和背面透射。本研究表明,片剂质量的增加会导致背面透射的时间延迟增加。这是由于固体部分的折射率比空气高。研究表明,透射率可用作片剂质量的间接测量,其均方根误差约为 1 毫克。研究还探讨了以高采集率(50 赫兹)测量片剂的可能性,并认为这是可行的。此外,先前的工作已经证明,正面反射的时间延迟允许同时评估片剂高度。所介绍的方法为在线监测片剂质量提供了可能性,是生产环境中高采样率下含量和含量均匀性策略的一部分。此外,由于可以同时评估片剂高度和质量,因此还可以在全面评估片剂物理属性的基础上对压制过程进行监测和控制。本文章由计算机程序翻译,如有差异,请以英文原文为准。
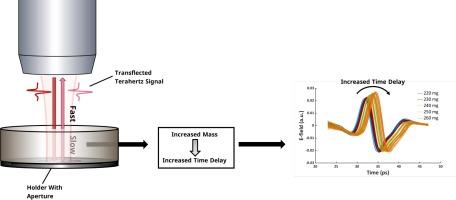
THz-TDS transflection measurements as a process analyser for tablet mass
Tablet content and content uniformity are essential for the market release of the drug product. For tablets, content and uniformity are determined by the weight ratio of active pharmaceutical ingredient in the tablet and the tablets’ total mass. Novel process analytical technology tools for the control of the ratio of the active pharmaceutical ingredient have been proposed and implemented, but more robust, sensitive, and fast sensors for the control of tablet mass are desirable. In the presented study terahertz time-domain spectroscopy (THz-TDS) is proposed as a potential process analyser for tablet mass. THz-TDS is based on pulsed terahertz signals, which are mapped in the time-domain. Thus, the signal amplitude and arrival time are recorded. THz-TDS measurements of a tablet with a reflection setup result in two signals – a frontside reflection and a backside transflection. The presented study demonstrates that an increase in the tablet mass results in an increase in the time delay of the backside transflection. This is a result of the high refractive index of the solid fraction compared to air. It is suggested that the time delay of the transflection can be used as an indirect measure of tablet mass for which root mean squared errors of around 1 mg were found. The potential to measure tablets at high acquisition rates (50 Hz) is explored and considered feasible. Additionally, it has been demonstrated in previous work that the time delay of the frontside reflection allows a simultaneous assessment of the tablet height. The presented methodology opens the possibility of in-line monitoring of tablet mass as part of a content and content uniformity strategy at high sampling rates in the production environment. Further, as tablet height and mass can be assessed simultaneously, monitoring and control of the compression process based on a comprehensive assessment of physical tablet attributes can also be envisioned.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
10.70
自引率
8.60%
发文量
951
审稿时长
72 days
期刊介绍:
The International Journal of Pharmaceutics is the third most cited journal in the "Pharmacy & Pharmacology" category out of 366 journals, being the true home for pharmaceutical scientists concerned with the physical, chemical and biological properties of devices and delivery systems for drugs, vaccines and biologicals, including their design, manufacture and evaluation. This includes evaluation of the properties of drugs, excipients such as surfactants and polymers and novel materials. The journal has special sections on pharmaceutical nanotechnology and personalized medicines, and publishes research papers, reviews, commentaries and letters to the editor as well as special issues.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


