Pegaharolines A - I,从 Peganum harmala L. 种子中提取的具有抗 HSV-2 病毒活性的结构新颖的吲哚生物碱。
IF 2.5
3区 医学
Q3 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
在对 HSV-2 病毒的抗病毒活性的引领下,以生物活性为指导,从 Peganum harmala 种子中分离出了 9 种结构新颖的吲哚生物碱,即 pegaharolines A - I(1-9)和 11 种已知的生物碱(10-20)。化合物 3 是一种不寻常的 6/5/5/5 双环吲哚生物碱,其特征是一个经典的双环吲哚单元通过一个螺旋原子(C-3)与另一个吡咯烷环融合。化合物 4 通过单晶 X 射线衍射被确定为一种新型吲哚生物碱,具有罕见的六环 6/5/6/5-6/6 环系统。化合物 5 和 6 是奇特的吲哚二聚体,具有罕见的八环支架碳骨架。化合物 1-6 共有六个外消旋体。大多数化合物对 HSV-2 具有不同程度的抗病毒活性。特别是化合物 1 的抗 HSV-2 活性(IC50 = 0.90 ± 0.10 μM)远远优于阳性对照(阿昔洛韦,IC50 = 1.12 ± 0.15 μM)。在这项研究中,从哈密瓜种子中发现抗HSV-2成分将有利于开发和利用这种植物的抗病毒药物产品。本文章由计算机程序翻译,如有差异,请以英文原文为准。
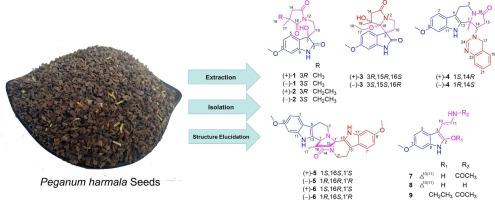
Pegaharolines A − I, structurally novel indole alkaloids with anti-HSV-2 virus activities from Peganum harmala L. seeds
Leading by the antiviral activities against HSV-2 virus, bioactivity-guided the fraction of crude alkaloids from seeds of Peganum harmala led to the isolation of nine structurally novel indole alkaloids, pegaharolines A − I (1–9), and 11 known ones (10−20). Compound 3 was an unusual 6/5/5/5 spirotetracyclic indole-derived alkaloids featuring a classic bicyclic indole unit fused with an additional pyrrolizine ring via a spiral atom (C-3). Compound 4 was determined as a novel indole alkaloid, characterized with a rare hexacyclic 6/5/6/5–6/6 ring system, by a single-crystal X-ray diffraction. Compounds 5 and 6 were peculiar indole dimers featuring with the rare carbon skeleton of an octacyclic scaffold. Compounds 1–6 were six racemates. Most compounds exhibited different levels of antiviral activities against HSV-2. Especially, the anti-HSV-2 activity of compound 1 (IC50 = 0.90 ± 0.10 μM) was much better than that of the positive control (acyclovir, IC50 = 1.12 ± 0.15 μM). In this study, the discovery of anti-HSV-2 components from the seeds of P. harmala, could benefit development and utilization of this plant in antiviral medicinal products.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Fitoterapia
医学-药学
CiteScore
5.80
自引率
2.90%
发文量
198
审稿时长
1.5 months
期刊介绍:
Fitoterapia is a Journal dedicated to medicinal plants and to bioactive natural products of plant origin. It publishes original contributions in seven major areas:
1. Characterization of active ingredients of medicinal plants
2. Development of standardization method for bioactive plant extracts and natural products
3. Identification of bioactivity in plant extracts
4. Identification of targets and mechanism of activity of plant extracts
5. Production and genomic characterization of medicinal plants biomass
6. Chemistry and biochemistry of bioactive natural products of plant origin
7. Critical reviews of the historical, clinical and legal status of medicinal plants, and accounts on topical issues.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


