血丝氨酰 tRNA 合成酶(srs-2)的特征。
IF 1.6
4区 医学
Q3 PARASITOLOGY
引用次数: 0
摘要
这项研究的目的是纯化和鉴定具有抗寄生虫疫苗潜力的重组蛋白。克隆了血清酰-tRNA合成酶(sss-2)的全长cDNA,它们分别来自Haemonchus contortus(HcSRS-2)和Teladorsagia circumcincta(TcSRS-2)。TcSRS-2 和 HcSRS-2 cDNA(1458bp)编码 486 个氨基酸的蛋白质,每个蛋白质在 SDS-PAGE 上呈一条约 55 kDa 的条带。蛋白质序列的多重比对显示,TcSRS-2 与 HcSRS-2 的同源性为 94%,与八种线虫的 SRS-2 的同源性为 76-93%,与麝的 SRS-2 的同源性为 68%。预测的三维结构显示,TcSRS-2 和 HcSRS-2 在整体结构上具有同源性,其结合位点和催化位点高度保守,而其他线虫 SRS-2 同源物的同功酶结合位点残基则略有不同。利用线虫和哺乳动物的 SRS-2 序列构建了一棵系统发生树。在大肠杆菌 AY2.4 菌株中表达并纯化可溶性 C 端 SRS-2 蛋白。重组 HcSRS-2 分析表明,重组酶具有活性且稳定。对 ATP 的 Km 和 Vmax 分别为 3.9 ± 1.0 μM 和 2.7 ± 0.1 μmoles min-1 mg-1 蛋白质。在酶联免疫吸附试验中,野外免疫羊(而非线虫免疫羊)血清和唾液中的抗体可识别重组 HcSRS-2 和 TcSRS-2。绵羊接触原生酶后产生的抗体能识别重组蛋白,这表明这两种蛋白具有相似的抗原性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
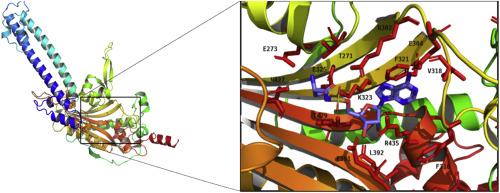
Characterisation of seryl tRNA synthetase (srs-2) in Haemonchus contortus and Teladorsagia circumcincta
The aim of the study was to purify and characterise recombinant proteins with the potential as an anti-parasite vaccine. Full-length cDNAs encoding seryl-tRNA synthetase (srs-2) were cloned from Haemonchus contortus (HcSRS-2) and Teladorsagia circumcincta (TcSRS-2). TcSRS-2 and HcSRS-2 cDNA (1458bp) encoded proteins of 486 amino acids, each of which was present as a single band of about 55 kDa on SDS-PAGE. Multiple alignments of the protein sequences showed homology of 94% between TcSRS-2 and HcSRS-2, 76–93% with SRS-2s of eight nematodes and 68% with Mus musculus SRS-2. The predicted three-dimensional structures revealed an overall structural homology of TcSRS-2 and HcSRS-2, highly conserved binding and catalytic sites, and minor differences in the tautomerase binding site residues in other nematode SRS-2 homologues. A phylogenetic tree was constructed using helminth and mammalian SRS-2 sequences. Soluble C-terminal SRS-2 proteins were expressed in Escherichia coli strain AY2.4 and purified. Recombinant HcSRS-2 assay shows that the recombinant enzyme was active and stable. The Km and Vmax for ATP were 3.9 ± 1.0 μM and 2.7 ± 0.1 μmol min−1 mg−1 protein, respectively. Antibodies in serum and saliva from field-immune, but not nematode-naïve, sheep recognised recombinant HcSRS-2 and TcSRS-2 in enzyme-linked immunosorbent assays. Recognition of the recombinant proteins by antibodies generated by exposure of sheep to the native enzyme indicates similar antigenicity of the two proteins.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Experimental parasitology
医学-寄生虫学
CiteScore
3.10
自引率
4.80%
发文量
160
审稿时长
3 months
期刊介绍:
Experimental Parasitology emphasizes modern approaches to parasitology, including molecular biology and immunology. The journal features original research papers on the physiological, metabolic, immunologic, biochemical, nutritional, and chemotherapeutic aspects of parasites and host-parasite relationships.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


