缺乏 BMAL1 会通过下调 ITPR2/3 导致口干和眼干。
IF 5.9
1区 医学
Q1 OPHTHALMOLOGY
引用次数: 0
摘要
分泌腺负责泪液和唾液的分泌,在维持眼部和口腔健康方面发挥着至关重要的作用。腺体分泌紊乱可由多种因素引起,包括与睡眠障碍有关的节律紊乱。然而,这些干扰的潜在机制在很大程度上仍未得到探索。我们证明,昼夜节律系统的核心成分 BMAL1 在调节分泌腺分泌方面起着关键作用。BMAL1 的缺失会诱导尖腺细胞出现空泡化和萎缩表型,随后导致细胞凋亡和腺体功能低下,但不会引起以局部炎性细胞浸润为特征的 Sjogren's 综合征。在机制上,BMAL1 直接调节 ITPR2 和 ITPR3 的转录,从而改变乳铁蛋白和溶菌酶的分泌。恢复 Bmal1 基因缺陷大鼠 ITPR2 和 ITPR3 的表达可有效缓解泪腺和腮腺分泌功能障碍的症状,并显著减轻节律紊乱大鼠的口干和眼干症状。这些发现强调了 BMAL1 在调节唾液腺和泪腺分泌中的重要作用,并为治疗与节律紊乱相关的口干和眼干提出了一种新的治疗方法。本文章由计算机程序翻译,如有差异,请以英文原文为准。
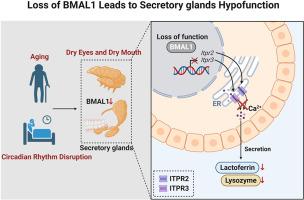
BMAL1 deficiency provokes dry mouth and eyes by down-regulating ITPR2/3
Secretory glands, responsible for tears and saliva production, play essential roles in maintaining ocular and oral well-being. Disruptions in gland secretion can arise from various factors, including rhythm disturbances associated with sleep disorders. However, the underlying mechanisms governing these disruptions remain largely unexplored. We demonstrate that BMAL1, a core component of the circadian system, plays a critical role in regulating secretory gland secretion. Loss of BMAL1 induces vacuolation and atrophy phenotypes in acinar cells, subsequently leading to cell apoptosis and gland hypofunction, but does not cause Sjogren's syndrome, which is characterized by localized inflammatory cell infiltration. Mechanically, BMAL1 directly modulates the transcription of ITPR2 and ITPR3, thereby altering the secretion of Lactoferrin and Lysozyme. Restoration of ITPR2 and ITPR3 expression in Bmal1-deficient rats effectively alleviated the symptoms of lacrimal and parotid glands secretory dysfunction and significantly reduced dry mouth and dry eye conditions in rhythm-disordered rats. These findings highlight the essential role of BMAL1 in regulating salivary and lacrimal gland secretion and suggest a novel therapeutic approach for treating dry mouth and dry eyes associated with rhythm disorders.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Ocular Surface
医学-眼科学
CiteScore
11.60
自引率
14.10%
发文量
97
审稿时长
39 days
期刊介绍:
The Ocular Surface, a quarterly, a peer-reviewed journal, is an authoritative resource that integrates and interprets major findings in diverse fields related to the ocular surface, including ophthalmology, optometry, genetics, molecular biology, pharmacology, immunology, infectious disease, and epidemiology. Its critical review articles cover the most current knowledge on medical and surgical management of ocular surface pathology, new understandings of ocular surface physiology, the meaning of recent discoveries on how the ocular surface responds to injury and disease, and updates on drug and device development. The journal also publishes select original research reports and articles describing cutting-edge techniques and technology in the field.
Benefits to authors
We also provide many author benefits, such as free PDFs, a liberal copyright policy, special discounts on Elsevier publications and much more. Please click here for more information on our author services.
Please see our Guide for Authors for information on article submission. If you require any further information or help, please visit our Support Center
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


