将米非司酮靶向输送到子宫内膜功能失调巨噬细胞以治疗子宫内膜异位症。
IF 9.4
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
摘要
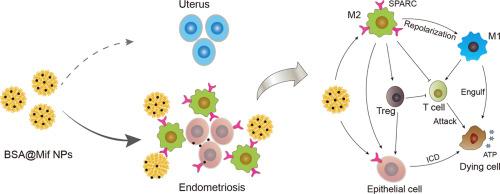
子宫内膜异位症严重影响着全球 6-10% 的育龄妇女,给临床带来了巨大挑战。子宫内膜细胞异位定植的过程与癌症有相似之处,而以非经典极化巨噬细胞为特征的免疫微环境功能失调在子宫内膜异位症的进展中起着关键作用。本研究以牛血清白蛋白(BSA)作为米非司酮的载体,开发了一种靶向纳米递送系统(BSA@Mif NPs)。BSA@Mif NPs 可通过 SPARC 受体选择性地靶向异位子宫内膜组织中高度富集的 M2 巨噬细胞。这种靶向策略在增加异位病灶的药物浓度的同时,最大限度地减少了药物在正常组织中的分布,从而降低了副作用。体外研究表明,BSA@Mif NPs 不仅能增强 M2 型巨噬细胞和异位子宫内膜细胞的细胞吸收,还能改善米非司酮对异位子宫内膜细胞的细胞毒性作用。此外,BSA@Mif NPs 还能有效诱导异位子宫内膜细胞的免疫原性细胞死亡(ICD),并使 M2 型巨噬细胞向 M1 表型重新极化,从而协同抑制异位子宫内膜细胞的生长。体内实验显示,BSA@Mif NPs 通过增加药物在子宫内膜异位组织中的蓄积和调节免疫微环境,在子宫内膜异位症小鼠中表现出显著的疗效。这种靶向仿生递送策略为开发基于现有药物的子宫内膜异位症特异性疗法提供了一种前景广阔的方法。意义说明:巨噬细胞在促进子宫内膜异位症发生和发展的免疫功能失调微环境中扮演着重要角色,可以作为开发免疫微环境调节策略的关键靶点,用于子宫内膜异位症的长期治疗。基于巨噬细胞和子宫内膜细胞中SPARC过表达及白蛋白生物安全性构建的白蛋白纳米颗粒,可通过增加异位子宫内膜中被动和主动介导的药物蓄积,重塑基于巨噬细胞调控的免疫微环境,从而实现子宫内膜异位症的靶向治疗。本研究具有以下启示:i) 利用纳米技术克服临床药物的固有缺陷是开发药物的另一种途径;ii) 开发基于巨噬细胞调控的微环境调控策略用于子宫内膜异位症的治疗是可行的。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Targeting delivery of mifepristone to endometrial dysfunctional macrophages for endometriosis therapy
Endometriosis seriously affects 6–10 % of reproductive women globally and poses significant clinical challenges. The process of ectopic endometrial cell colonization shares similarities with cancer, and a dysfunctional immune microenvironment, characterized by non-classically polarized macrophages, plays a critical role in the progression of endometriosis. In this study, a targeted nano delivery system (BSA@Mif NPs) was developed using bovine serum albumin (BSA) as the carrier of mifepristone. The BSA@Mif NPs were utilized to selectively target M2 macrophages highly enriched in ectopic endometrial tissue via the SPARC receptor. This targeting strategy increases drug concentration at ectopic lesions while minimizing its distribution to normal tissue, thereby reducing side effects. In vitro studies demonstrated that BSA@Mif NPs not only enhanced the cellular uptake of M2-type macrophages and ectopic endometrial cells but also improved the cytotoxic effect of mifepristone on ectopic endometrial cells. Furthermore, the BSA@Mif NPs effectively induced immunogenic cell death (ICD) in ectopic endometrial cells and repolarized M2-type macrophages toward the M1 phenotype, resulting in a synergistic inhibition of ectopic endometrial cell growth. In vivo experiments revealed that BSA@Mif NPs exhibited significant therapeutic efficacy in endometriosis-bearing mice by increasing drug accumulation in the endometriotic tissues and modulating the immune microenvironment. This targeted biomimetic delivery strategy presents a promising approach for the development of endometriosis-specific therapies based on existing drugs.
Statement of significance
Macrophages play an essential role in immune dysfunctional microenvironment promoting the occurrence and progression of endometriosis and can be a crucial target for developing immune microenvironment regulation strategies for the unmet long-term management of endometriosis. The albumin nanoparticles constructed based on SPARC overexpression in macrophages and endometrial cells and albumin biosafety can achieve the targeted therapy of endometriosis by increasing the passive- and active-mediated drug accumulation in ectopic endometrium and remodeling the immune microenvironment based on macrophage regulation. This study has the following implications: i) overcoming the inherent shortcomings of clinical drugs by nanotechnology is an alternative way of developing medication; ii) developing microenvironment modulation strategies based on macrophage regulation for endometriosis management is feasible.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Acta Biomaterialia
工程技术-材料科学:生物材料
CiteScore
16.80
自引率
3.10%
发文量
776
审稿时长
30 days
期刊介绍:
Acta Biomaterialia is a monthly peer-reviewed scientific journal published by Elsevier. The journal was established in January 2005. The editor-in-chief is W.R. Wagner (University of Pittsburgh). The journal covers research in biomaterials science, including the interrelationship of biomaterial structure and function from macroscale to nanoscale. Topical coverage includes biomedical and biocompatible materials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


