用于三阴性乳腺癌治疗的双重免疫刺激CD73抗体-聚合细胞毒性药物复合物。
IF 9.4
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
摘要
由于三阴性乳腺癌(TNBC)具有转移倾向,因此其治疗面临巨大挑战。一个关键的障碍在于抑制性免疫微环境,这种环境会助长肿瘤进展。本研究介绍了一种采用双重免疫刺激CD73抗体-聚合细胞毒性药物复合物(αCD73-PLG-MMAE)的方法。这种复合物旨在靶向根除 TNBC,同时通过免疫原性细胞死亡(ICD)和干扰腺苷信号通路等机制调节肿瘤免疫。通过增强抗肿瘤免疫反应,这种策略为治疗 TNBC 和减少转移提供了一种非常有效的手段。利用αCD73和Fc-III-4C之间的亲和力,αCD73与聚(L-谷氨酸)(PLG)接枝Fc结合肽(Fc-III-4C)和Val-Cit-PAB-monomethyl auristatin E(MMAE)结合合成了复合物。αCD73 可选择性地靶向肿瘤细胞和免疫抑制细胞上的 CD73 分子,从而抑制腺苷途径。同时,Val-Cit-PAB-MMAE 被酪蛋白酶 B 激活后,会触发 MMAE 的选择性释放,从而诱导肿瘤细胞的 ICD。在 4T1 肿瘤模型中,与 IgG-PLG-MMAE 相比,αCD73-PLG-MMAE 能显著提高药物在肿瘤中的蓄积 4.13 倍,从而抑制肿瘤的生长和转移。此外,它还能协同增强αPD-1的抗肿瘤作用,使肿瘤抑制率达到92%,而单独使用αPD-1的抑制率仅为21%。因此,这项研究提出了一种治疗 TNBC 的开创性策略,强调了靶向免疫调节在癌症治疗中的潜力。意义声明:抗体药物结合物(ADC)疗法有望治疗三阴性乳腺癌(TNBC)。然而,目前的 ADCsacituzumab govitecan 无法克服腺苷在这种 "冷肿瘤 "特有的抑制性免疫微环境中的关键作用。在这里,我们提出了一种双重免疫刺激复合物αCD73-PLG-MMAE,它专门针对TNBC,通过免疫原性细胞死亡(ICD)和干扰腺苷信号通路等机制调节肿瘤免疫。因此,它能通过细胞毒性药物杀死肿瘤细胞,全面调节免疫抑制,恢复持久的免疫反应。本研究提出了一种具有免疫调节和免疫拮抗作用的抗体-聚合药物复合物,为 TNBC 的治疗提供了新的思路。本文章由计算机程序翻译,如有差异,请以英文原文为准。
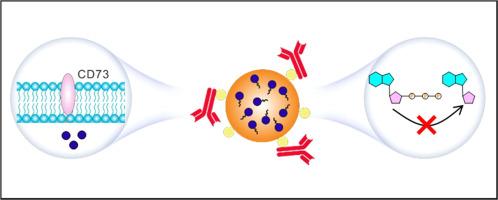
Dual immunostimulatory CD73 antibody-polymeric cytotoxic drug complex for triple negative breast cancer therapy
Treatment of triple-negative breast cancer (TNBC) poses significant challenges due to its propensity for metastasis. A key impediment lies in the suppressive immune microenvironment, which fosters tumor progression. This study introduces an approach employing a dual immune-stimulatory CD73 antibody-polymeric cytotoxic drug complex (αCD73-PLG-MMAE). This complex is designed for targeted eradication of TNBC while modulating tumor immunity through mechanisms such as immunogenic cell death (ICD) and interference with the adenosine signaling pathway. By enhancing antitumor immune responses, this strategy offers a highly effective means of treating TNBC and mitigating metastasis. The complex is synthesized by combining αCD73 with poly(L-glutamic acid) (PLG) grafted Fc binding peptides (Fc-III-4C) and Val-Cit-PAB-monomethyl auristatin E (MMAE), exploiting the affinity between αCD73 and Fc-III-4C. αCD73 selectively targets CD73 molecules on both tumor and immune suppressive cells, thereby inhibiting the adenosine pathway. Meanwhile, Val-Cit-PAB-MMAE, activated by cathepsin B, triggers selective release of MMAE, inducing ICD in tumor cells. In a 4T1 tumor model, αCD73-PLG-MMAE significantly enhances drug accumulation in tumors by 4.13-fold compared to IgG-PLG-MMAE, leading to suppression of tumor growth and metastasis. Furthermore, it synergistically augments the antitumor effects of αPD-1, resulting in a tumor inhibition rate of 92 % as compared to 21 % with αPD-1 alone. This study thus presents a pioneering therapeutic strategy for TNBC, emphasizing the potential of targeted immunomodulation in cancer treatment.
Statement of significance
Antibody-drug conjugate (ADC) therapy holds promise for treating triple-negative breast cancer (TNBC). However, the current ADC, sacituzumab govitecan, fails to overcome the crucial role of adenosine in the suppressive immune microenvironment characteristic of this "cold tumor". Here, we present a dual immune-stimulatory complex, αCD73-PLG-MMAE, which targets TNBC specifically and modulates tumor immunity through mechanisms such as immunogenic cell death (ICD) and interference with the adenosine signaling pathway. Thus, it kills tumor cells with cytotoxic drugs, comprehensively regulates immunosuppression, and restores a durable immune response. This study proposes an antibody-polymeric drug complex with immunomodulatory and immunoagonist roles, offering new insights into TNBC treatment.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Acta Biomaterialia
工程技术-材料科学:生物材料
CiteScore
16.80
自引率
3.10%
发文量
776
审稿时长
30 days
期刊介绍:
Acta Biomaterialia is a monthly peer-reviewed scientific journal published by Elsevier. The journal was established in January 2005. The editor-in-chief is W.R. Wagner (University of Pittsburgh). The journal covers research in biomaterials science, including the interrelationship of biomaterial structure and function from macroscale to nanoscale. Topical coverage includes biomedical and biocompatible materials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


