YAP 可促进全局 mRNA 翻译,从而在饥饿状态下促进致癌生长。
IF 9.5
2区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
是相关蛋白(YAP)和具有PDZ结合基调的转录协同激活因子(TAZ)在稳态过程中的干细胞/祖细胞扩增中发挥着重要作用,而它们的失调往往会导致组织过度生长。在这里,我们发现YAP的激活足以克服血清饥饿引起的全局蛋白质合成限制,使细胞在不利的环境中仍能维持增殖和存活。从机理上讲,YAP/TAZ选择性地促进了mTORC1依赖的含有5'末端寡嘧啶(5'TOP)基序的mRNA的翻译,最终增加了细胞多聚体的含量。有趣的是,DNA损伤诱导转录本4(DDIT4)是mTORC1的负调控因子,它在血清饥饿时上调,但被YAP/TAZ抑制。DDIT4足以抑制葡萄膜黑色素瘤细胞的翻译和转化潜能,而这些细胞往往因G蛋白突变而对血清无反应。我们的研究结果揭示了蛋白质合成作为YAP/TAZ诱导的致癌转化的关键模式的重要作用,并表明了靶向mTORC1或翻译治疗YAP/TAZ驱动的恶性肿瘤的潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
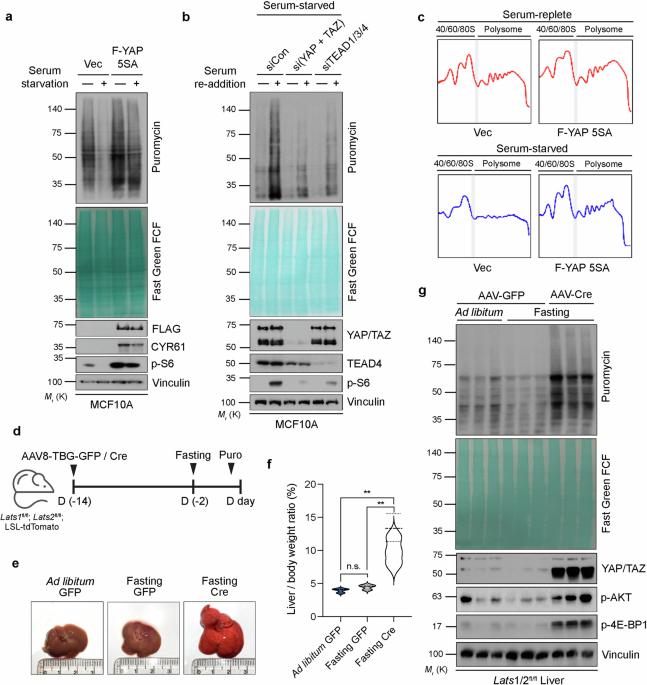
YAP promotes global mRNA translation to fuel oncogenic growth despite starvation
Yes-associated protein (YAP) and transcriptional co-activator with PDZ-binding motif (TAZ) play fundamental roles in stem/progenitor cell expansion during homeostasis, and their dysregulation often leads to tissue overgrowth. Here, we show that YAP activation is sufficient to overcome the restriction of global protein synthesis induced by serum starvation, enabling cells to sustain proliferation and survival despite an unfavorable environment. Mechanistically, YAP/TAZ selectively promoted the mTORC1-dependent translation of mRNAs containing 5′ terminal oligopyrimidine (5′TOP) motifs, ultimately increasing the cellular polysome content. Interestingly, DNA damage-inducible transcript 4 (DDIT4), a negative regulator of mTORC1, was upregulated by serum starvation but repressed by YAP/TAZ. DDIT4 was sufficient to suppress the translation and transformative potential of uveal melanoma cells, which are often serum unresponsive due to G protein mutations. Our findings reveal a vital role for protein synthesis as a key modality of YAP/TAZ-induced oncogenic transformation and indicate the potential for targeting mTORC1 or translation to treat YAP/TAZ-driven malignancies. This research investigates how cells manage their size and proliferation by coordinating two signaling pathways, Hippo and mTOR. As these pathways are fundamental for normal development, their dysregulation results in numerous diseases, including many cancers. In particular, the study aims to understand how YAP and TAZ—effectors of the Hippo pathway—influence mTOR-mediated protein synthesis in cells, a previously unclear process. Surprisingly, our findings show that YAP and TAZ can maintain active protein synthesis even when cells are deprived of nutrients in both cultured cells and mice. Since self-sufficiency in growth signals is a key hallmark of cancer, targeting this axis could serve as a novel and effective therapeutic strategy. This summary was initially drafted using artificial intelligence, then revised and fact-checked by the author.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Experimental and Molecular Medicine
医学-生化与分子生物学
CiteScore
19.50
自引率
0.80%
发文量
166
审稿时长
3 months
期刊介绍:
Experimental & Molecular Medicine (EMM) stands as Korea's pioneering biochemistry journal, established in 1964 and rejuvenated in 1996 as an Open Access, fully peer-reviewed international journal. Dedicated to advancing translational research and showcasing recent breakthroughs in the biomedical realm, EMM invites submissions encompassing genetic, molecular, and cellular studies of human physiology and diseases. Emphasizing the correlation between experimental and translational research and enhanced clinical benefits, the journal actively encourages contributions employing specific molecular tools. Welcoming studies that bridge basic discoveries with clinical relevance, alongside articles demonstrating clear in vivo significance and novelty, Experimental & Molecular Medicine proudly serves as an open-access, online-only repository of cutting-edge medical research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


