真皮层常驻巨噬细胞应对炎症挑战的复原力。
IF 9.5
2区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
皮肤是由组织驻留巨噬细胞(TRMs)构成的复杂屏障器官,在防御、稳态和组织修复中发挥着关键作用。本综述探讨了真皮驻留巨噬细胞在不同炎症环境中的功能、其胚胎起源及其长期自我更新能力。我们强调了真皮TRMs的M2样表型及其在血管周围和神经元周围龛位中的特殊功能。它们与 2 型免疫细胞、IL-10 等自分泌细胞因子之间的相互作用,以及它们对凋亡细胞的吞噬清除,都被探讨为 M2 类真皮 TRM 自我维持和发挥功能的机制。总之,我们需要在小鼠模型与人体研究之间架起一座桥梁,从而有可能通过靶向 TRMs 促进皮肤免疫或抑制皮肤病理学。本文章由计算机程序翻译,如有差异,请以英文原文为准。
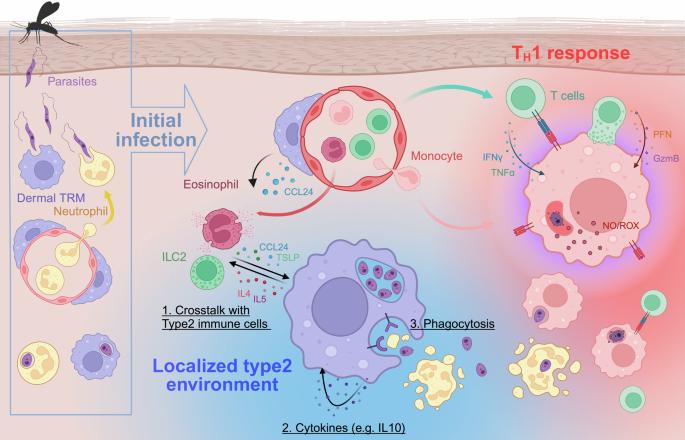
Resilience of dermis resident macrophages to inflammatory challenges
The skin serves as a complex barrier organ populated by tissue-resident macrophages (TRMs), which play critical roles in defense, homeostasis, and tissue repair. This review examines the functions of dermis resident TRMs in different inflammatory settings, their embryonic origins, and their long-term self-renewal capabilities. We highlight the M2-like phenotype of dermal TRMs and their specialized functions in perivascular and perineuronal niches. Their interactions with type 2 immune cells, autocrine cytokines such as IL-10, and their phagocytic clearance of apoptotic cells have been explored as mechanisms for M2-like dermal TRM self-maintenance and function. In conclusion, we address the need to bridge murine models with human studies, with the possibility of targeting TRMs to promote skin immunity or restrain cutaneous pathology. Our skin is more than just a physical shield; it’s a complex immune organ, filled with specialized cells like macrophages. In a detailed review, researchers explore these macrophages, focusing on their adaptability and function maintenance in the skin. This study synthesizes findings how macrophages interact with other immune cells, respond to inflammatory triggers, and contribute to tissue repair and homeostasis. The findings show that these macrophages can remain anti-inflammatory, even when faced with infections that usually trigger a strong immune response. They achieve this through various mechanisms, including interactions with specific immune cells that support their anti-inflammatory state, and engaging in processes that promote tissue repair without increasing inflammation. The researchers conclude that understanding these mechanisms opens new possibilities for treating skin diseases by targeting or mimicking the ways these macrophages control inflammation and support healing. This summary was initially drafted using artificial intelligence, then revised and fact-checked by the author.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Experimental and Molecular Medicine
医学-生化与分子生物学
CiteScore
19.50
自引率
0.80%
发文量
166
审稿时长
3 months
期刊介绍:
Experimental & Molecular Medicine (EMM) stands as Korea's pioneering biochemistry journal, established in 1964 and rejuvenated in 1996 as an Open Access, fully peer-reviewed international journal. Dedicated to advancing translational research and showcasing recent breakthroughs in the biomedical realm, EMM invites submissions encompassing genetic, molecular, and cellular studies of human physiology and diseases. Emphasizing the correlation between experimental and translational research and enhanced clinical benefits, the journal actively encourages contributions employing specific molecular tools. Welcoming studies that bridge basic discoveries with clinical relevance, alongside articles demonstrating clear in vivo significance and novelty, Experimental & Molecular Medicine proudly serves as an open-access, online-only repository of cutting-edge medical research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


