肾脏成纤维细胞参与了与端粒功能障碍相关的肾脏纤维化的纤维化变化。
IF 9.5
2区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
与慢性肾脏病(CKD)相关的肾小管间质纤维化是一个全球性的医疗保健问题。我们以前曾报道过端粒过短和功能失调会导致肾间质纤维化;然而,与端粒功能失调相关的肾纤维化的细胞起源目前尚不清楚。我们在小鼠的短期和长期实验中,通过特异性地删除肾脏成纤维细胞中编码端粒结合因子的 Trf1 基因来诱导端粒功能障碍,以确定成纤维细胞在肾脏纤维化中的作用。在肾脏成纤维细胞中短期缺失 Trf1 不足以引发肾脏纤维化,但足以诱发炎症反应、ECM 沉积、细胞周期停滞、纤维生成和血管稀疏。然而,在成纤维细胞中长期持续缺失 Trf1 会导致肾脏纤维化,并伴有尿白蛋白-肌酐比值(uACR)升高和小鼠存活率下降。这些细胞反应导致巨噬细胞向肌成纤维细胞转化(MMT)、内皮细胞向间质转化(EndMT)和部分上皮细胞向间质转化(EMT),当成纤维细胞中的Trf1缺失在小鼠整个生命周期中持续存在时,最终导致人道终点(HEP)肾脏纤维化。我们的研究结果有助于人们更好地理解端粒功能失调在导致肾脏纤维化的脆性改变中的作用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
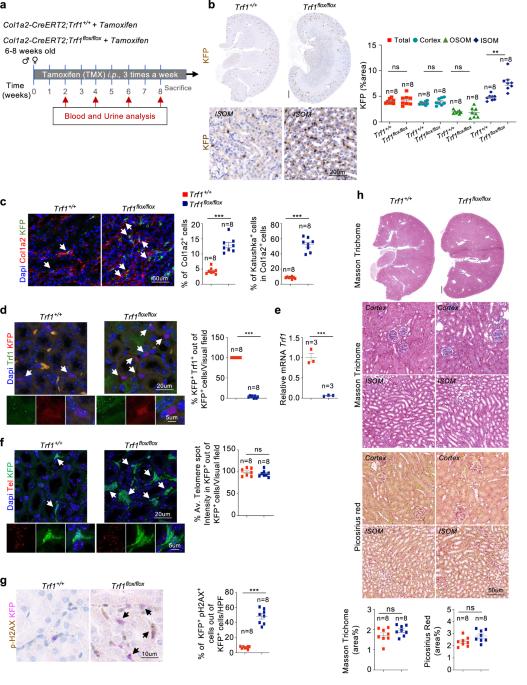
Renal fibroblasts are involved in fibrogenic changes in kidney fibrosis associated with dysfunctional telomeres
Tubulointerstitial fibrosis associated with chronic kidney disease (CKD) represents a global health care problem. We previously reported that short and dysfunctional telomeres lead to interstitial renal fibrosis; however, the cell-of-origin of kidney fibrosis associated with telomere dysfunction is currently unknown. We induced telomere dysfunction by deleting the Trf1 gene encoding a telomere-binding factor specifically in renal fibroblasts in both short-term and long-term life-long experiments in mice to identify the role of fibroblasts in renal fibrosis. Short-term Trf1 deletion in renal fibroblasts was not sufficient to trigger kidney fibrosis but was sufficient to induce inflammatory responses, ECM deposition, cell cycle arrest, fibrogenesis, and vascular rarefaction. However, long-term persistent deletion of Trf1 in fibroblasts resulted in kidney fibrosis accompanied by an elevated urinary albumin-to-creatinine ratio (uACR) and a decrease in mouse survival. These cellular responses lead to the macrophage-to-myofibroblast transition (MMT), endothelial-to-mesenchymal transition (EndMT), and partial epithelial-to-mesenchymal transition (EMT), ultimately causing kidney fibrosis at the humane endpoint (HEP) when the deletion of Trf1 in fibroblasts is maintained throughout the lifespan of mice. Our findings contribute to a better understanding of the role of dysfunctional telomeres in the onset of the profibrotic alterations that lead to kidney fibrosis. Chronic kidney disease, a condition that can lead to kidney failure and death, affects millions worldwide. It’s characterized by kidney fibrosis, a process where healthy kidney tissue becomes scar tissue. The exact cells that start this process were unknown. Researchers studied the role of a protein called TRF1, which protects the ends of chromosomes, in kidney fibroblasts, cells that make connective tissue. They removed TRF1 from these cells in mice and observed the effects. Results showed that removing TRF1 from fibroblasts increased fibrosis, inflammation, and kidney damage. Specifically, fibroblasts without TRF1 were more likely to change into myofibroblasts, leading to more scar tissue. Study concluded that TRF1 is vital in preventing kidney fibrosis by keeping fibroblasts healthy. This finding improves our understanding of CKD and suggests potential treatments targeting fibroblasts and TRF1 to fight kidney fibrosis. This summary was initially drafted using artificial intelligence, then revised and fact-checked by the author.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Experimental and Molecular Medicine
医学-生化与分子生物学
CiteScore
19.50
自引率
0.80%
发文量
166
审稿时长
3 months
期刊介绍:
Experimental & Molecular Medicine (EMM) stands as Korea's pioneering biochemistry journal, established in 1964 and rejuvenated in 1996 as an Open Access, fully peer-reviewed international journal. Dedicated to advancing translational research and showcasing recent breakthroughs in the biomedical realm, EMM invites submissions encompassing genetic, molecular, and cellular studies of human physiology and diseases. Emphasizing the correlation between experimental and translational research and enhanced clinical benefits, the journal actively encourages contributions employing specific molecular tools. Welcoming studies that bridge basic discoveries with clinical relevance, alongside articles demonstrating clear in vivo significance and novelty, Experimental & Molecular Medicine proudly serves as an open-access, online-only repository of cutting-edge medical research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


