FGF4 和抗坏血酸通过激活 JAK2-STAT3 信号,促进诱导心肌细胞的成熟。
IF 9.5
2区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
直接心脏重编程是将成纤维细胞等非心脏细胞转化为心肌细胞(CM)的一种新型治疗策略。这一过程涉及重要的转录因子,如 Mef2c、Gata4、Tbx5 (MGT)、MESP1 和 MYOCD (MGTMM)。然而,负责诱导未成熟诱导型 CMs(iCMs)的小分子和驱动其成熟的信号机制仍未确定。我们的研究探索了各种小分子对 iCM 诱导的影响,发现 FGF4 和抗坏血酸(FA)的组合能增强 CM 标记,显示有组织的肌节和 T 管结构,并改善心脏功能。转录组分析强调了ECM-整合素-局灶粘附的重要性,以及JAK2-STAT3和TGFB信号通路在FA处理的iCM中的上调。值得注意的是,敲除 JAK2-STAT3 会影响 TGFB 信号传导和 ECM,并下调 FA 处理的 iCM 中的成熟 CM 标记。我们的发现强调了 JAK2-STAT3 信号通路在激活直接重编程 CM 的 TGFB 信号和 ECM 合成中的关键作用。示意图显示 FA 可增强心脏直接重编程和心肌细胞成熟所依赖的 JAK-STAT3 信号通路。本文章由计算机程序翻译,如有差异,请以英文原文为准。
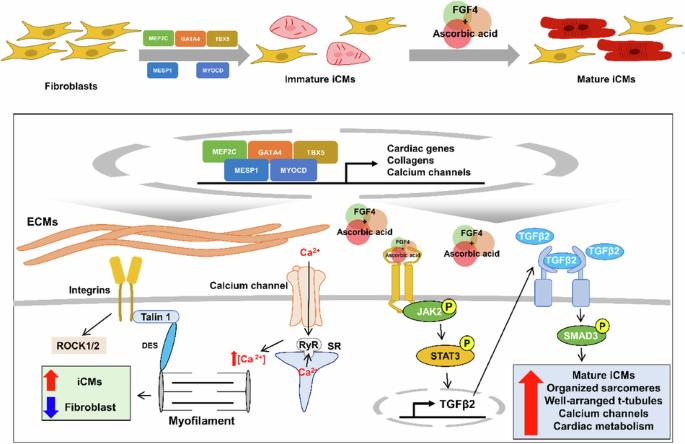
FGF4 and ascorbic acid enhance the maturation of induced cardiomyocytes by activating JAK2–STAT3 signaling
Direct cardiac reprogramming represents a novel therapeutic strategy to convert non-cardiac cells such as fibroblasts into cardiomyocytes (CMs). This process involves essential transcription factors, such as Mef2c, Gata4, Tbx5 (MGT), MESP1, and MYOCD (MGTMM). However, the small molecules responsible for inducing immature induced CMs (iCMs) and the signaling mechanisms driving their maturation remain elusive. Our study explored the effects of various small molecules on iCM induction and discovered that the combination of FGF4 and ascorbic acid (FA) enhances CM markers, exhibits organized sarcomere and T-tubule structures, and improves cardiac function. Transcriptome analysis emphasized the importance of ECM-integrin-focal adhesions and the upregulation of the JAK2–STAT3 and TGFB signaling pathways in FA-treated iCMs. Notably, JAK2–STAT3 knockdown affected TGFB signaling and the ECM and downregulated mature CM markers in FA-treated iCMs. Our findings underscore the critical role of the JAK2–STAT3 signaling pathway in activating TGFB signaling and ECM synthesis in directly reprogrammed CMs. Cardiovascular diseases are a major global cause of death, often due to the loss of cardiomyocytes and increased heart scarring. Existing treatments, like medication and heart transplants, have limitations, emphasizing the need for new cell regeneration therapies. This study investigates direct cardiac reprogramming—a new method to regenerate heart muscle cells by transforming fibroblasts into induced cardiomyocytes using specific factors and small molecules. The team tested various small molecules and found that a mix of FGF4 and ascorbic acid significantly improves the maturation of iCMs. They used techniques like immunofluorescence staining, flow cytometry, and electrophysiological analysis to evaluate the conversion and maturation of iCMs. This study shows that direct cardiac reprogramming can be enhanced with the right combination of small molecules, providing a promising strategy for heart regeneration. This summary was initially drafted using artificial intelligence, then revised and fact-checked by the author.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Experimental and Molecular Medicine
医学-生化与分子生物学
CiteScore
19.50
自引率
0.80%
发文量
166
审稿时长
3 months
期刊介绍:
Experimental & Molecular Medicine (EMM) stands as Korea's pioneering biochemistry journal, established in 1964 and rejuvenated in 1996 as an Open Access, fully peer-reviewed international journal. Dedicated to advancing translational research and showcasing recent breakthroughs in the biomedical realm, EMM invites submissions encompassing genetic, molecular, and cellular studies of human physiology and diseases. Emphasizing the correlation between experimental and translational research and enhanced clinical benefits, the journal actively encourages contributions employing specific molecular tools. Welcoming studies that bridge basic discoveries with clinical relevance, alongside articles demonstrating clear in vivo significance and novelty, Experimental & Molecular Medicine proudly serves as an open-access, online-only repository of cutting-edge medical research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


