镍催化的远程 C(sp3)-H 功能化(使用 "遛链 "策略
IF 51.7
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
金属催化剂沿烷基侧链的动态转移--通常被称为 "链行"--开辟了新的逆合成可能性,使未活化的 C(sp3)-H 位点得以功能化。由于镍络合物在形成 sp3 结构方面的多功能性,以及其氧化还原性可促进单电子或双电子反应歧管,因此在链烷基化策略中使用镍络合物最近获得了相当大的发展势头。本综述讨论了这些过程在合成工作中可能产生的相关性和影响,包括适当时的机理考虑。其中特别强调了利用镍催化链式反应的潜力来解决原本难以实现的转化问题的最新发现,包括链式反应与光氧化催化或电化学活化等其他新方法的结合。本文章由计算机程序翻译,如有差异,请以英文原文为准。
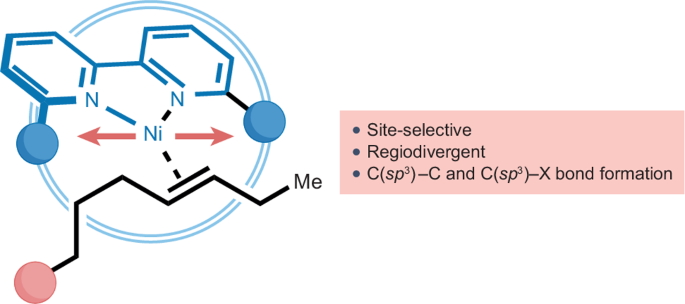
Ni-catalysed remote C(sp3)–H functionalization using chain-walking strategies
The dynamic translocation of a metal catalyst along an alkyl side chain — often coined as ‘chain-walking’ — has opened new retrosynthetic possibilities that enable functionalization at unactivated C(sp3)–H sites. The use of nickel complexes in chain-walking strategies has recently gained considerable momentum owing to their versatility for forging sp3 architectures and their redox promiscuity that facilitates both one-electron or two-electron reaction manifolds. This Review discusses the relevance and impact that these processes might have in synthetic endeavours, including mechanistic considerations when appropriate. Particular emphasis is given to the latest discoveries that leverage the potential of Ni-catalysed chain-walking scenarios for tackling transformations that would otherwise be difficult to accomplish, including the merger of chain-walking with other new approaches such as photoredox catalysis or electrochemical activation. Ni-catalysed chain-walking blossomed as an effective synthetic tool to functionalize C(sp3)–H bonds in hydrocarbon chains. This Review provides a detailed overview of the most recent advances in this field, focusing on site-selective and regioselective manipulations at previously out-of-reach C(sp3)–H sites.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature reviews. Chemistry
Chemical Engineering-General Chemical Engineering
CiteScore
52.80
自引率
0.80%
发文量
88
期刊介绍:
Nature Reviews Chemistry is an online-only journal that publishes Reviews, Perspectives, and Comments on various disciplines within chemistry. The Reviews aim to offer balanced and objective analyses of selected topics, providing clear descriptions of relevant scientific literature. The content is designed to be accessible to recent graduates in any chemistry-related discipline while also offering insights for principal investigators and industry-based research scientists. Additionally, Reviews should provide the authors' perspectives on future directions and opinions regarding the major challenges faced by researchers in the field.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


