硅氧烷包裹脂质纳米颗粒的组合设计可增强细胞内处理能力,实现组织特异性 mRNA 治疗递送
IF 38.1
1区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
摘要
利用脂质纳米颗粒(LNPs)系统输送信使 RNA(mRNA)以实现组织特异性靶向具有巨大的治疗潜力。然而,可电离脂质(类脂质)的结构特征如何影响其靶向细胞和器官的能力仍不清楚。在这里,我们设计了一类结构各异的硅氧烷基可离子化脂质,并配制了硅氧烷包合 LNPs(SiLNPs),以控制体内 mRNA 向小鼠肝脏、肺脏和脾脏的递送。硅氧烷分子能增强 mRNA-LNPs 的细胞内化,提高其内逸能力,从而增强其 mRNA 递送功效。利用器官特异性 SiLNPs 传递基因编辑机制,我们在野生型小鼠的肝脏以及转基因 GFP 和路易斯肺癌(LLC)肿瘤小鼠的肺部实现了强大的基因敲除。此外,我们还利用肺靶向 Si5-N14 LNPs 释放血管生成因子,显示了病毒感染引起的肺损伤的有效恢复。我们设想,我们的 SiLNPs 将有助于 mRNA 疗法的临床转化,用于下一代组织特异性蛋白质替代疗法、再生医学和基因编辑。本文章由计算机程序翻译,如有差异,请以英文原文为准。
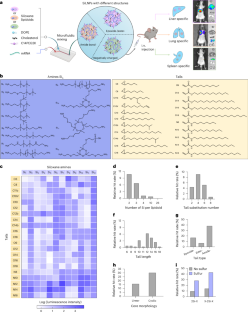

Combinatorial design of siloxane-incorporated lipid nanoparticles augments intracellular processing for tissue-specific mRNA therapeutic delivery
Systemic delivery of messenger RNA (mRNA) for tissue-specific targeting using lipid nanoparticles (LNPs) holds great therapeutic potential. Nevertheless, how the structural characteristics of ionizable lipids (lipidoids) impact their capability to target cells and organs remains unclear. Here we engineered a class of siloxane-based ionizable lipids with varying structures and formulated siloxane-incorporated LNPs (SiLNPs) to control in vivo mRNA delivery to the liver, lung and spleen in mice. The siloxane moieties enhance cellular internalization of mRNA-LNPs and improve their endosomal escape capacity, augmenting their mRNA delivery efficacy. Using organ-specific SiLNPs to deliver gene editing machinery, we achieve robust gene knockout in the liver of wild-type mice and in the lungs of both transgenic GFP and Lewis lung carcinoma (LLC) tumour-bearing mice. Moreover, we showed effective recovery from viral infection-induced lung damage by delivering angiogenic factors with lung-targeted Si5-N14 LNPs. We envision that our SiLNPs will aid in the clinical translation of mRNA therapeutics for next-generation tissue-specific protein replacement therapies, regenerative medicine and gene editing. mRNA delivery through LNPs targeting specific organs holds great clinical potential, but it remains unclear how the structure of the lipidoids in the LNPs controls organ tropism. Here the authors direct in vivo delivery of siloxane-based LNPs via structural alteration of the ionizable structure of the constituting lipidoids.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature nanotechnology
工程技术-材料科学:综合
CiteScore
59.70
自引率
0.80%
发文量
196
审稿时长
4-8 weeks
期刊介绍:
Nature Nanotechnology is a prestigious journal that publishes high-quality papers in various areas of nanoscience and nanotechnology. The journal focuses on the design, characterization, and production of structures, devices, and systems that manipulate and control materials at atomic, molecular, and macromolecular scales. It encompasses both bottom-up and top-down approaches, as well as their combinations.
Furthermore, Nature Nanotechnology fosters the exchange of ideas among researchers from diverse disciplines such as chemistry, physics, material science, biomedical research, engineering, and more. It promotes collaboration at the forefront of this multidisciplinary field. The journal covers a wide range of topics, from fundamental research in physics, chemistry, and biology, including computational work and simulations, to the development of innovative devices and technologies for various industrial sectors such as information technology, medicine, manufacturing, high-performance materials, energy, and environmental technologies. It includes coverage of organic, inorganic, and hybrid materials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


